(94) pH-Dependent Optical Properties of Nanographenes
Mayu Nakamura, Kaito Fukushima, Aoto Ikeda, Kaori Miyazaki, Shun Yamaguchi,
Yudai Ono, Takeharu Haino, Naoto Nishiyama,* and Ryo Sekiya,* ChemPhysChem,
2026, 27, e202500746.
DOI:10.1002/cphc.202500746.

Abstract:Nanographenes (NGs) produced by oxidative cleavage of carbons exhibit pH-dependent optical properties. We performed UV-vis and PL measurements on size-separated NGs across various pH levels and observed their halochromic behaviors in both the visible and near-infrared (NIR) regions. The absorption spectra exhibited a two-step change. The initial change is attributed to the deprotonation and protonation of the carboxy groups, and the latter is likely due to the aggregation and disaggregation of NGs. Notably, no significant shifts in the absorption and PL bands were observed, indicating that the deprotonation of the carboxy groups has a minimal impact on the electronic structures of NGs in both their ground and excited states. This implied that the effect of the deprotonation was limited to the edge moieties. Computational calculations suggested edge-to-edge and edge-to-surface π-π* transitions in the visible and NIR regions for a deprotonated model NG. Thus, a plausible explanation for the halochromic behavior of NGs involves the disappearance and appearance of these transitions upon the protonation and deprotonation of the carboxy groups. These changes affect the absorption in the visible and NIR regions, resulting in the halochromic behavior of NGs.
(93) Supramolecular Aggregates of Amide- and Urea-Functionalized Nanographene
Haruka Moriguchi, Ryo Sekiya,* and Takeharu Haino,* ChemistryEurope, 2025,
3, e2500015.
DOI:10.1002/ceur.202500015
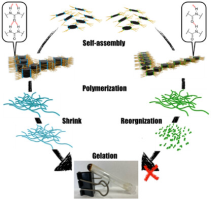
Abstract:Controlling the morphology of supramolecular nanographene(NG) aggregates is challenging. This study confirms that amideand urea-functionalized NG undergo self-assembly to form supramolecular aggregates with a morphology that depends on the incorporated functional group. Amide-functionalized NG forms stacked aggregates, whereas urea-functionalized NG organizes into network polymers. These distinct morphologies suggest that amide groups drive NG stacking, whereas urea groups support NG vertically and horizontally, likely owing to differences in the strengths of single and bifurcated N─H/O hydrogen bonds. Moreover, the functional group incorporated into NG influences the gelation properties of the system. Among the two tested systems, only urea-functionalized NG formed organogels, possibly because urea–urea hydrogen bonds, enable solvent-molecule trapping inside the network polymers formed in these NG systems. Thus, hydrogen bonds can regulate the morphology and function of supramolecular NG aggregates.
(92) Chirality Generation on Carbon Nanosheets by Chemical Modification
Ryo Sekiya,* Saki Arimura, Haruka Moriguchi, and Takeharu Haino,* Nanoscale,
2025, 17, 774-787.
DOI:10.1039/d4nr02952f
Abstract:Chirality is an intriguing property of molecules, and an exciting area
of study involves the generation of chirality in nanographene (NGs), also
known as graphene quantum dots. Unlike those synthesized through stepwise
carbon-carbon bond formation by organic reactions (bottom-up method), NGs
are obtained by cutting parent carbons (top-down method) pose challenges
in precisely regulating their three-dimensional structures by post-synthesis.
This includes the incorporation of non-hexagonal rings and helicene-like
structures in carbon frameworks. Currently, edge functionalization is the
only method for generating chirality in NGs produced by the top-down method.
While various chiral NGs have been synthesized through organic methods,
examples of chemical modification remain rare due to limited structural
information and the substantial size of NGs. However, these problems can
be mitigated by disclosing the structures of NGs, particularly their edge
structures. This minireview focuses on recently published papers that address
the structural characterization of NGs and their chirality generation by
edge modification. Comparing these NGs with those synthesized by organic
synthesis will help to develop reasonable strategies for creating sophisticated
chiral NGs. We hope this mini-review contributes to the advancement of
NG-organic hybrid materials.
(91) Assessment of Edge Modification of Nanographene
Ryo Sekiya* and Takeharu Haino,* ChemPhysChem, 2024, 25, e202400792(Front Cover).
DOI:10.1002/cphc.202400792
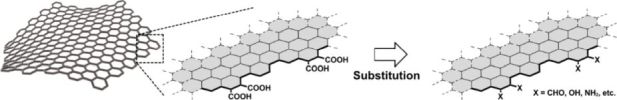
Abstract:Carboxy groups on the edges of nanographene (NG) enable functionalization for realizing NG-organic hybrid materials. Therefore, assessment of the edge-functionalization of the electronic structures of NGs is valuable for the rational design of functional carbon materials. In this study, the structures of model NGs comprising 174 carbon atoms with armchair edges and various functional groups at the edges were computed. To achieve the greatest possible similarity between the computed structure and the real one, the carbon framework was designed based on experimental observations. The functional groups can be accessed via suitable chemical reactions. The computations predicted that although the conversion of carboxyl groups with electron-withdrawing/donating groups influences the orbital energies, the HOMO-LUMO (H-L) gap is not significantly affected, except in a few cases. Among the evaluated examples, π-extension had the greatest influence on the H-L gap. Interestingly, for the Pd2+-coordinated NG, the participation of the low-lying LUMO localized on Pd2+ in the surface-to-metal transitions seemingly narrowed the H-L gap, and a surface-to-ligand transition was observed.
(90) Conformation Regulation of Trisresorcinarene Directed by Cavity Solvation
Daisuke Shimoyama,† Ryo Sekiya,† Shoichiro Inoue, Naoyuki Hisano, Shin-ichi
Tate, and Takeharu Haino,* Chem. Eur. J., 2024, 30, e202402922 (Front Cover).
DOI:10.1002/chem.202402922
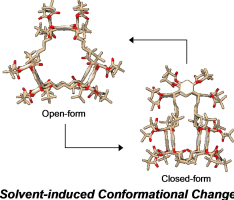
Abstract:This compound is a synthetic macrocycle comprising three pivaloyl-protected resorcinarene units connected by six pentylene chains. We conducted a conformational study using 1H-NMR, X-ray diffraction (XRD), and computational analyses. The macrocycle adopts two conformers, one open, the other closed. The ratio of the open to closed forms depended on the solvent used. Only the open form existed in [D8]toluene, both forms coexisted in [D6]benzene, and the closed form was the major conformer in [D1]chloroform. The benzene-solvated open form observed in the solid state suggests that cavity solvation by solvent molecules directs the open form. The open form was the major or only conformer in [D10]o- and [D10]m-xylene and [D12]mesitylene, whereas the closed form was the major conformer in [D6]acetone. The open and closed forms were equally populated in [D10]p-xylene, suggesting that the size, shape, and dimensions of the solvent molecules most likely influenced the conformation of the protected trisresocinarene.
(89) Selective Encapsulation of Carboxylic Acid Dimeric Pairs within a
Size-regulable Resorcinarene-based Hemicarcerand
Kentaro Harada, Yudai Ono, Ryo Sekiya, and Takeharu Haino,* Chem. Commun.,
2024, 60, 6603-6606 (Inside Front Cover).
DOI:10.1039/d4cc00699b
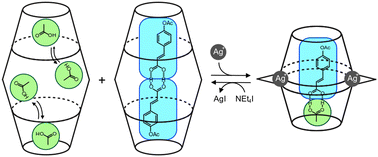
Abstract: A cavity within a resorcinarene-based hemicarcerand was contracted and expanded through conformational changes induced by the complexation and decomplexation, allowing self-sorting of homo- and heterodimeric carboxylic acid pairs.
(88) Kinetic Resolution of Secondary Alcohols Catalyzed at the Exterior
of Chiral Coordinated Capsules
Kentaro Harada,† Ryo Sekiya,† Takeharu Haino,* Chem. Eur. J., 2024, 30,
e202304244 (Front Cover).
DOI:10.1002/chem.202304244
Press release (広島大学), 科学新聞
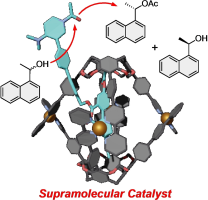
Abstract: Confined spaces inside molecular hosts can function as reaction vessels. However, this concept significantly limits the scope of reactants. When the exterior of molecular hosts is used instead, we can ease the restriction because reactants are not necessary to be trapped inside molecular hosts, although studies along this line have not been reported. As a proof-of-concept of enantioselective reactions at the exterior of chiral molecular hosts, we utilized host-guest complexes of enantiomerically enriched Cu-coordinated capsules and guests possessing a catalytic center to realize the kinetic resolution of secondary alcohols. Under suitable reaction conditions, a selectivity factor of 2.6 was realized, demonstrating that the reactions occur at the exterior of the capsules. A series of experiments indicated that the substituents on the 2,2’-bipyridyl arms and the alkyl chains on the lower rim contributed to the enantioselectivity of the reactions.
(87) Application of Exciton Coupling for Characterization of Nanographene
Edge
Ryo Sekiya* and Takeharu Haino,* ChemPhysChem, 2024, 25, e202300740 (Front Cover).
DOI:10.1002/cphc.202300740
Interview
DOI:10.1002/cphc.202400134
Abstract:The structural characterization of nonstoichiometric nanographene (NG)-organic hybrid materials is usually difficult. The number of substituents on the edge and their arrangements are frequently questioned but are difficult to answer. Since the number of functional groups is closely related to the distance between the nearest neighbors (dISD), the extraction of dISD from spectroscopic data could provide important information on their structural characterization. We show that exciton coupling, which is a theoretical prediction of the absolute structures of discrete molecules, is a possible candidate to address this issue. The comparison of the calculated CD spectra of the chiral chromophores extracted from the model NG edge with the observed edge spectra indicated a dISD of ca. 8 Å; this corresponded to substitution on every other armchair edge. Furthermore, an up-up-down-down alternate orientation was found to be a possible edge structure. Although the procedure was limited to NGs carrying chiral substituents, our method could facilitate the detailed structural characterization of NG-organic hybrid materials.
(86) Intermediate Color Emission via Nanographenes with Organic Fluorophores
Saki Arimura, Ikuya Matsumoto, Ryo Sekiya,* and Takeharu Haino,* Angew.
Chem. Int. Ed., 2024, 63, e202315508 (Frontispiece).
DOI:10.1002/anie.202315508
Press release (広島大学), 日刊工業新聞

Abstract: Photoluminescence (PL) color can be tuned by mixing fluorophores emitting the three primary colors in an appropriate ratio. When color tuning is achieved on a single substrate, we can simplify device structures. We demonstrated that nanographenes (NGs), which are graphene fragments with a size of tens of nanometers, could be utilized as carriers of fluorophores. The addition of red- and blue-light-emitting fluorophores on the edge successfully reproduced the purple light. The relative PL intensities of the fluorophores could be regulated by the excitation wavelength, enabling multicolor emission between blue and red light. Owing to the triphenylamine units of the fluorophores, the NGs showed PL enhancement due to aggregation. This characteristic was valuable for the fabrication of solid polymer materials. Specifically, the functionalized NGs can be dispersed into polyvinylidene difluoride. The resultant polymer films emitted red, blue, and purple color. Our study demonstrated the potential applicability of NGs for fluorophore carriers capable of reproducing intermediate colors of light.
(85) Molecular Recognition Process in Resorcinarene-based Coordination
Capsules
Kentaro Harada,† Ryo Sekiya,† Takeharu Haino,* Chem. Eur. J., 2023, 29,
e202302581.
DOI:10.1002/chem.202302581
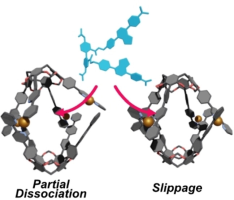
Abstract:Cu and Ag capsules can take up various organic molecules. Their molecular recognition possibly involves partial dissociation and slippage. We investigated molecular recognition processes in the Cu and Ag capsules by CD and 1H NMR spectroscopy and employed 4,4’-diacetoxy biphenyl carrying two benzothiadiazole groups as a probe. CD and 1H NMR measurements reveal that the host-guest complexation proceeds under second-order reactions and that these capsules undergo the partial dissociation to take up the probe in [D1]chloroform and [D8]THF. The slippage also contributes to host-guest complexation for a Cu capsule that carries p-methoxyphenyl groups on the 2,2’-bipyridiyl arms. DFT calculations suggest that π/π stacking interactions between the electron-rich p-methoxyphenyl group and the electron-poor 2,2’-bipyridyl arm elongate the capsule, allowing the guest to access the cavity without the partial dissociation.
(84) Induction of Chirality on Nanographenes
Saki Arimura, Ikuya Matsumoto, Shohei Nishitani, Ryo Sekiya,* and Takeharu
Haino,* Chem. Asian J., 2023, 18, e202300126.
DOI:10.1002/asia.202300126
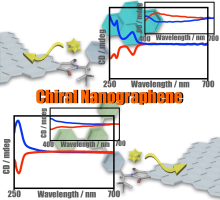
Abstract: Chirality induction is an important topic in studies of nanographenes (NGs). We report chirality enhancements of NGs through postsynthetic chemical modifications of NGs with pyrene and m-terphenyl groups. These substituents were installed into N-(p-bromophenylethyl)imides on the edges of the NGs with Pd-catalyzed cross-coupling reactions. Circular dichroism (CD) spectra demonstrated that these bulky substituents improved the induced CD signal of the NGs compared to those previously reported and suggested that they induced the opposite chirality. Density functional theory calculations indicated possible edge structures for the NGs and indicated that π/π and CH/π interactions among the neighboring substituents influenced the orientations of the imides. These imides distorted the edges, and the distorted edges eventually generated the chiral environments of the NGs. The interactions among the substituents are, therefore, likely to allow detection of the CD signals in the visible region and induction of the opposite chirality.
(83) Effects of Edge Functionalization of Nanographenes with Small Aromatic
Systems
Shusaku Takahashi, Ryo Sekiya,* and Takeharu Haino,* ChemPhysChem, 2023,
24, e202300066.
DOI:10.1002/cphc.202300066
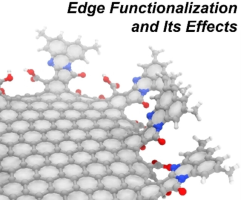
Abstract: Regulation of the physical properties of nanographenes (NGs) by edge functionalization is an active research area. We conducted a computational study of the effects of edge functionalization on the physical properties of NGs. The computed NGs were models of experimentally obtained NGs and composed of a C174 carbon framework with one to four 3,5-dimethylnaphthalene units on the edge. The effects were assessed structurally, magnetically, and electronically by the least square planarity index, harmonic oscillator model of aromaticity, nucleus-independent chemical shift, and HOMO–LUMO (H–L) gaps. Density functional theory calculations indicate that although the structures of the model NGs are not very sensitive to edge functionalization, but the magnetic and electronic properties are. The installed substituents narrowed the H−L gap and induced a redshift of the photoluminescence (PL) band by the π conjugation between NG and the substituent. These results are consistent with the extension of the absorption band and the redshift of the PL bands of the experimentally modified NGs. Furthermore, the calculations confirmed the contribution of the charge transfer character to the absorption spectra.
(82) Substituent-Induced Supramolecular Aggregates of Edge Functionalized
Nanographenes
Haruka Moriguchi, Ryo Sekiya,* and Takeharu Haino,* Small, 2023, 19, 2207475.
DOI:10.1002/smll.202207475
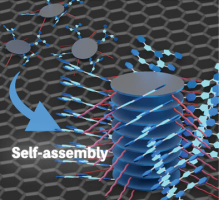
Abstract: Precisely controlled molecular assemblies often display intriguing morphologies
and/or functions arising from their structures. The application of the
concept of the self-assembly for controlling the aggregation of nanographenes
(NGs) is challenging. The title NGs are those carrying both long alkyl
chains and tris(phenylisoxazolyl)benzene (TPIB) on the edge. The former
group secures the affinity of NGs for organic solvents, and the latter
group drives the 1D arrangement of NGs through the interactions between
the TPIB units. The concentration-dependent and temperature variable 1H
NMR, UV–vis, and PL spectra demonstrate the aggregation of NGs in 1,2-dichloroethane,
and the aggregation is controllable by the regulation of the solvent polarity.
AFM images give the stacked structures of the NGs, and these aggregates
turn out to be network polymeric structures at a high concentration. These
observations demonstrate that the synergy of the face-to-face interactions
between the surfaces and the interactions between the TPIB units are effective
for controlling the self-assembly of the NGs.
(81) Computational Study on the Structures of Nanographenes with Various
Edge Functionalities
Shusaku Takahashi, Ryo Sekiya,* and Takeharu Haino,* ChemPhysChem, 2023,
24, e202200465.
DOI:10.1002/cphc.202200465

Abstract: Computational studies have often been carried out on hydrogen-terminated nanographenes (NGs). These structures are, however, far from those deduced from experimental observations, which have suggested armchair edges with two carboxy groups on the edges as dominant. We conducted computational studies on NGs consisting of C42, C60, C78, C96, C142, and C174 carbon atoms with hydrogen, carboxy, and N-methyl imide-terminated armchair edges. DFT calculations inform distorted basal planes and similar HOMO-LUMO gaps, indicating that the edge oxidation and functionalization do not very influence the electronic structure. Comparison of observed UV-vis spectra of carboxy- and N-octadecyl chain terminated NGs with calculated spectra of model NGs informs the contribution of π-π* transitions on the basal plane to the absorptions in the visible region. A dimeric structure of NG and octadecyl-installed NG demonstrate that both the distorted basal planes and the steric contacts among the functional groups widen the surface-to-surface distance thereby allowing the invasion of solvent molecules between the surfaces. This picture is consistent with the improved solubility of edge-modified NGs.
(80) Metal Nanoparticles on Lipophilic Nanographenes
Shusaku Takahashi, Ryo Sekiya,* and Takeharu Haino, *Angew. Chem. Int.
Ed., 2022, 61, e202205514(Hot Paper, Frontispiece).
DOI:10.1002/anie.202205514
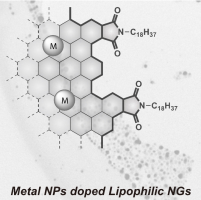
Utilization of graphitic domains on nanographenes (NGs) for anchoring metal nanoparticles (NPs) can open the door for their applications in catalysis. Reported examples employ hydrophilic graphene oxides as substrates, making it difficult to coexist with organic substrates in organic solvents. The title NGs are metal NP-doped lipophilic NGs (M-NG1). Various metal cations form NPs with a diameter of a few to tens of nanometers on the basal plane. Owing to the lipophilic nature of NG1, M-NG1 is prepared by the reduction of the basal plane with sodium in THF followed by the addition of metal salts. Au-NPs on NG1 allow anchoring an organic thiol carrying an anthracene fluorophore, which is a proof of concept of composite materials utilizing the surface of NGs. The assessment of the catalytic function of Pd-NG1 reveals that chlorobenzene and bromobenzene yield a coupling product, and fluorobenzene also undergoes the reactions, demonstrating the catalytic function of Pd-NG1.
(79) Chirality Induction on a Coordination Capsule for Circularly Polarized
Luminescence
Kentaro Harada,† Ryo Sekiya,† and Takeharu Haino,* Angew. Chem. Int. Ed.,
2022, 61, e202209340.
DOI:10.1002/anie.202209340
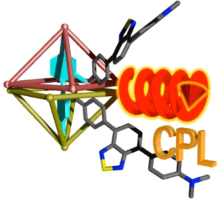
Abstract: y utilizing a confined space inside a coordination capsule consisting of achiral components, we achieve trimeric structures composed of acetic acid and 2,3-disubstituted tartaric acid derivatives. Steric and electronic interactions between the substituents on the tartaric acid and 2,2′-bipyridyl arms of the capsule permit the transfer of the chirality of the tartaric acid to the capsule, resulting in diastereoenrichment of the host-guest complexes of up to 92 % de. The chiral templates can be washed away with diethyl ether, leaving an enantiomerically enriched capsule. The resulting capsule biases the dynamic axial chirality of a 4,4′-diacetoxybiphenyl guest carrying benzothiadiazole units, demonstrating guest-to-capsule and capsule-to-guest chirality transfer. The induced chirality on the bound guest enables it to emit circularly polarized luminescence in the NIR region, demonstrating the application of induced chirality for confined spaces for the generation of chiroptical properties.
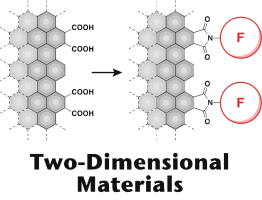
(78) Integration of Nanographenes and Organic Chemistry – Toward Nanographene-based
Two-Dimensional Materials
Ryo Sekiya* and Takeharu Haino,* ChemPhysChem, 2022, 23, e202200311 (Very Important Paper).
DOI:10.1002/cphc.202200311
Interview
DOI:10.1002/cphc.202200738
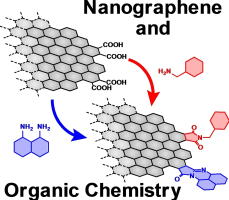
Abstract: Graphene and its relatives have received considerable attention from the fields of physics and chemistry since the isolation of pristine graphene sheets. Nanographenes (NGs) are graphene fragments that are a few to tens of nanometers in diameter. Compared to graphene and its relatives, such as graphene oxides, NGs can be handled more easily, and their large π surface and oxygen functional groups on the edge allow postsynthetic modifications. The study of NGs is gradually shifting from the development of synthetic procedures to postsynthetic modification. From the structural point of view, NGs can be regarded as two-dimensional carbon polymers. Their unique structures and affinity for organic molecules make NGs excellent scaffolds for two-dimensional materials, which are now an important topic in organic and polymer chemistry. In this conceptual article, we introduce the position of NGs from the perspective of two-dimensional substances and briefly review both the structural features of NGs and the effects of functionalization on their physical properties. These are valuable when producing reasonable strategies for their postsynthetic modifications.
(77) Chirality Induction in a Hydrophilic Metallohelicate
Masayuki Morie,† Ryo Sekiya,† and Takeharu Haino,* Chem. Asian J., 2022,
17, e202200275.
DOI:10.1002/asia.202200275
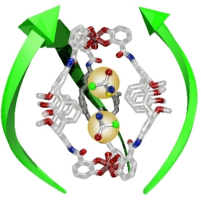
Abstract: The title compound is a water-soluble triply stranded metallohelicate 1Fe composed of TEG-chain-installed calix[4]arene-based bis-bidentate ligands and Fe3+ cations. This compound can incorporate up to two molecules of small chiral cations (G1 and G2). Interestingly, G1 shows positive cooperativity for host-guest complexation, whereas G2 exhibits noncooperativity, despite having a greater affinity for 1Fe than G1. Density functional theory (DFT) calculations indicate the metallohelicate cavity expands upon encapsulation of the first guest. This conformational change facilitates the entrapment of the second guest and explains why the interaction parameters of G1 and G2 are larger than 1, despite electrostatic repulsion between the guests. The DFT calculation predicts an intermolecular interaction between the phenyl groups of G1 in the cavity, whereas no such interaction is found for G2. This difference can explain the distinct molecular recognition of G1 and G2. CD spectroscopy shows that both (R)-G1 and (R)-G2 induce (M)-helicity in 1Fe and vice versa. DFT calculations suggest that the chirality of the guests is transmitted to 1Fe via CH/O hydrogen bonds between the guest and the C=O bond on the calix[4]arene strand. The CD intensity of 1Fe is nonlinear in the ee% of (R)- and (S)-G1, indicating that the presence of the first guest increases the affinity of 1Fe toward a second guest with the same chirality due to preorganization by the first guest.
(76) Electrochromism of Nanographenes in the Near-Infrared Region
Ikuya Matsumoto, Ryo Sekiya,* Hiroji Fukui, Ren-De Sun, and Takeharu Haino,*
Angew. Chem. Int. Ed., 2022, 61, e202200291(Hot Paper, Back side cover).
DOI:10.1002/anie.202200291
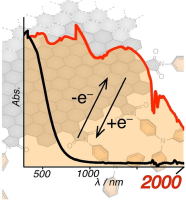
Abstract:Nanographene (NG) is a potential candidate for organic EC materials because of its large π-conjugated system, chemical stability, absorption band covering the visible region, and tunable optical properties by postsynthetic modification. We show that NGs carrying redox-active triphenylamine (TPA) units covalently linked to the NG edge function as EC materials in the NIR region. The hybrid materials can be obtained by the installation of TPA units onto the NG edge and display changes in the absorption spectrum in the NIR region extending to a wavelength of over 2000 nm upon one-electron oxidation and reduction at low potentials (<1.1 V). Time-dependent unrestricted density functional theory calculation of a model NG at the UB3LYP/6-31G(d,p) level of theory suggests that a narrow energy gap between the basal plane and the oxidized TPA unit is responsible for the observed EC function in the NIR region.
(75) Resorcinarene-based Supramolecular Capsules – Supramolecular Functions
and Applications (Review Article)
Ryo Sekiya, Kentaro Harada, Natsumi Nitta, and Takeharu Haino,* Synlett,
2022, 33, 518-530.
DOI:0.1055/a-1679-8141
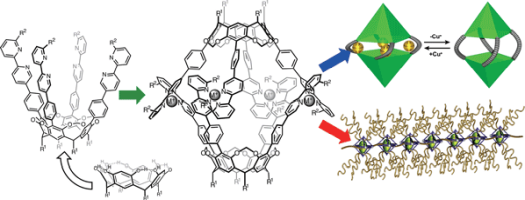
Abstract: A resorcinarene is a synthetic macrocycle consisting of four resorcinol molecules covalently linked by methylene bridges. The interannular bridges produce a cavitand that has a bowl-shaped structure. We have developed supramolecular capsules through Ag(I) or Cu(I) coordination-driven self-assembly of cavitands possessing 2,2′-bipyridyl arms in their upper rims. The self-assembled capsules accommodate various molecular guests and supramolecular assemblies possessing acetoxy groups. The host–guest chemistry of the molecular capsules has been applied in the fabrication of supramolecular polymers. This account describes recent developments in the supramolecular chemistry of resorcinarene-based coordination capsules and provides a brief history of resorcinarene-based capsules and related capsules.
(74) Nanographene – A Scaffold of Two-Dimensional Materials
Ryo Sekiya* and Takeharu Haino,* Chem. Rec., 2022, 22, e202100257
DOI:10.1002/tcr.202100257
Abstract: Substances can be divided into 0D to 3D species based on the number of repeating units (atom, ion, and molecule) and their arrangements in space (point, linear, layer, and solid). Discrete substances belong to 0D species, polymers are examples of 1D species, and molecular crystals are 3D species. Most of the substances belong to one of these species. On the other hand, those categorized into 2D species wherein the repeating units organize a layer are less explored. 2D species have a surface and edges. The incorporation of these structural features into a molecular design can realize multifunctionalized systems that are difficult to achieve by conventional organic synthesis. The development of 2D species is, therefore, the frontier of organic, inorganic, and polymer chemistry. Nanographenes (NGs) are suitable scaffolds for realizing 2D species due to several factors, such as chemical stability and oxygen-containing functional groups on the surface and on the edge, allowing postsynthetic modifications. Our group has utilized NGs with tens of nanometers in diameters for developing 2D species. Carboxy groups on the edge enable us to install various substituents into NGs, offering NG-based functional materials. These studies demonstrate that the integration of NGs with organic chemistry can widen the scope of their applications other than optical materials that are a main application of NGs. We introduce our recent studies on the development of NG-based functional materials realized by postsynthetic modifications. We hope that this account will contribute to the development of the chemistry of 2D species.
(73) Synthesis and Conformational Characteristics of Calix[4]arene Oligomers
Masauki Morie, Ryo Sekiya, and Takeharu Haino, Bull. Chem. Soc. Jpn, 2021,
94, 2792-2799(Selected Paper, Back Cover).
DOI:10.1246/bcsj.20210287

Abstract: Lower- and/or upper-rim functionalization of calix[4]arene realized a variety of calix[4]arene systems. Compared to these monomeric calix[4]arenes, covalently linked calix[4]arene oligomers have not been studied well. Calix[4]arene oligomers can be utilized as building blocks of supramolecular complexes as well as for the synthesis of calix[4]arene polymers. This background motivated us to develop synthetic procedures for calix[4]arene oligomers and to conduct conformational analysis of these oligomers. We produce oligomers ranging from the monomer to the pentamer. The coupling of the pentamer with 2,3-dibenzyloxy-1,4-benzenedicarboxylic acid can access a decamer and oligomers. NMR measurements, X-ray crystal structure analysis, and computational studies demonstrate that calix[4]arene oligomers can regulate their length by changing the conformations of the calix[4]arene cores.
(72) Chemical Modification of Nanographenes and Their Functions
Ryo Sekiya* and Takeharu Haino,* J. Synth. Org. Chem. Jpn., 2021, 79, 743-753.
DOI:0.5059/yukigoseikyokaishi.79.743
Abstract: Nanographenes, known as graphene quantum dots, are graphene fragments with a few to tens of nanometers. Due to the quantum size effect, nanographenes have bandgaps with a few eV and display photoemission in the visible region. These properties make nanographenes attractive research targets. Nanographenes can be obtained by the oxidative cleavage of parent carbons such as graphite and carbon fiber with strong oxidants. We have employed a mixture of concentrated sulfuric acid and 60% nitric acid as oxidants. Although as obtained products are the nonstoichiometric mixture of nanographenes, their size separation with dialysis membranes offers nanographenes with narrower lateral size distribution that displays excitation-wavelength-independent photoemission. Nanographenes carry a large number of carboxylic acid groups at the edge that can be utilized for chemical functionalization by organic synthesis. An example is the installation of 4-propargyloxy benzylamine at the edge. The reactive triple bonds on the resulting nanographenes allow to conduct the Cu(I) catalyzed click reactions, yielding edge-functionalized nanographenes. The click reaction of the nanographene with 1st, 2nd, and 3rd generation dendritic wedges produces white-light-emitting nanographenes and the installation of both long alkyl chains and ureidopyrimidinone groups, which form self-complementary four-fold hydrogen bonds, into the edge drives the self-assembly of the functionalized nanographenes to organize polymer-like structures that function as organogelators. This article focuses on the chemical modification of nanographenes by organic synthesis.
(71) Self-Assembly of Nanographenes
Ikuya Matsumoto,† Ryo Sekiya,† and Takeharu Haino,* Angew. Chem. Int. Ed.,
2021, 60, 12706-12711(Selected Paper, Back Cover).
DOI:10.1002/anie.202101992
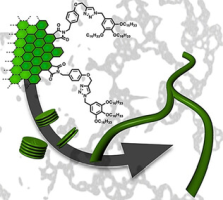
Abstract:Suitably decorated small aromatic systems can organize stacked structures that display interesting properties arising from their unique morphologies. Although nanographenes produced by top-down methods have graphitic domains and can in principle be applied for such supramolecular systems, to our knowledge, no such example has been reported thus far. This is partly because of their limited solubility in organic solvents and partly because of their wide lateral size distribution. To realize nanographene-based supramolecular aggregates, nanographenes carrying alkyl chains with narrow lateral size distributions are employed. We find that the nanographenes undergo self-assembly and that self-assembly is regulated by concentration, solvent polarity, temperature, and sonication. Optical measurements and AFM images indicate that stacked structures are possible candidates for aggregates. A molecular mechanics calculation models the interactions in the aggregates. The nanographenes showed concentration-dependent morphologies on mica, stacked structures at low concentrations and polymer-like network structures on mica at higher concentrations.
(70) Translational Isomers of N-Sulfonylated[3]catenane: Synthesis and
Isomerization
Hajime Iwamoto,* Yuki Ishizu, Eietsu Hasegawa, Ryo Sekiya, and Takeharu
Haino, Chem. Commun., 2021, 57, 1915–1918.
DOI:10.1039/D0CC07720H
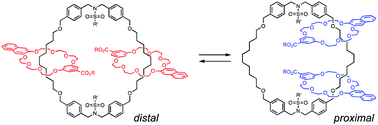
Abstract: N-Sulfonylated [3]catenanes, which exist as two translational isomers, were synthesized. The X-ray crystal structure of the distal isomer of [3]catenane, which has higher symmetry, revealed hydrogen bonds involving the carboxylic acid moieties on the terminal rings. The thermodynamic parameters of the isomerization revealed that this hydrogen bonding influenced the isomerization process.
(69) Calix[4]arene-Based Triple Stranded Metallohelicate in Water
Masayuki Morie,† Ryo Sekiya,† and Takeharu Haino,* Chem. Asian. J., 2021,
16, 49–55(Cover Picture).
DOI:10.1002/asia.202001154
Abstract: The title complex is a triple-stranded metallohelicate organized by the self-assembly of 5,17-difunctionalized calix[4]arenes and metal cations with octahedral coordination geometry. Due to hydrophilic triethylene glycol chains on the lower rim of the calix[4]arene, the metallohelicate can encapsulate cationic guests in water. NMR and UV-vis titration experiments reveal that the metallohelicate captures a pyridinium guest with an alanine derivative to form a host-guest complex with a host-guest ratio of 1 : 1. CD spectroscopy confirms the bias of the P- and M-helical sense of the metallohelicate by the captured guest. The metallohelicate captures two molecules of dicationic N,N’-dimethyl-DABCO and monocationic N-methyl quinuclidine, exhibiting a positive allosteric effect. 1H NMR titration experiments indicate that the bound guests are in close proximately to the aromatic rings of the ligands. Molecular mechanics calculations based on the UV-vis and NMR observations suggest that the first guest preorganizes the conformation of the metallohelicate to facilitate access of the second guest to the cavity.
(68) Nanographenes from Distinct Carbon Sources
Ikuya Masyumoto,† Ryo Sekiya,† and Takeharu Haino,* Bull. Chem. Soc. Jpn.,
2021, 94, 1394–1399 (Selected Paper, Back Cover).
DOI:10.1246/bcsj.20200381
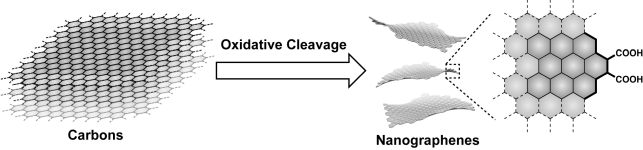
Abstract: This article reports production protocols for nanographenes and the effect of the reaction conditions on their structures and optical properties. These fundamental studies are of value for exploring suitable reaction conditions for the production of nanographenes with desirable properties. Graphite, finely crushed graphite powders, and artificial graphite, all of which are commercially available, are employed. Nanographenes are produced by the acid-assisted oxidative cleavage of the parent carbons followed by neutralization and deionization. The use of dialysis membranes for the size separation of nanographenes offers nanographenes with a specific size distribution, thereby allowing their structures and optical properties to be compared. Experiments demonstrate that small amounts of acids (60 ml of conc. H2SO4 and 20 mL of 60% HNO3) and oxidation for 12 h promotes a more efficient and cost-effective production of nanographenes from 2 g of a carbon source. The functionalization of the nanographene edges with p-propargyloxybenzyl amine confirms that the armchair edge with two carboxy groups is the dominant edge structure, irrespective of the carbon source.
(67) Folding and Unfolding of Acetoxy Group-Terminated Alkyl Chain Conformations
inside a Size-Regulable Hemicarcerand
Kentaro Harada,† Ryo Sekiya,† and Takeharu Haino,* J. Org. Chem., 2021,
86, 4440-4447 (Supplementary Journal Cover).
DOI:10.1021/acs.joc.0c02833
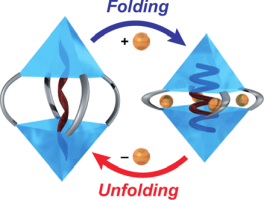
Abstract: A resorcinarene-based hemicarcerand, which consists of two cavitands covalently linked to each other by four alkyl chains, allows structural expansion and contraction by demetalation and metalation of Cu(I) cations with a size change of approximately 12 Å. This metal-mediated switching of the two states regulates the conformations of acetoxy group-terminated alkyl chains. A guest binding study reveals the encapsulation of heptyl to undecyl chains in metal-free and Cu(I)-coordinated capsules. The chemical shifts of the acetoxy groups of the bound guests are the same in the metal-free capsule, while those in the Cu(I)-coordinated one differ from each other. This indicates that the metal-free capsule regulates its size to the bound guests, while the bound guests adopt their conformations to the cavity of the Cu(I)-coordinated capsules. 1H NMR measurements and molecular mechanics calculations suggest that the bound guests have extended conformations in the metal-free capsule, while the Cu(I)-coordinated capsule forces the bound guests to adopt folded conformations. The presence of folded conformations is supported by the conformational study with structurally similar capsules and a nonsymmetric guest, allowing us to observe nuclear Overhauser effects stemming from the folded conformations of the guest in the cavity.
(66) Bluish-white-light Nanographenes Developed by Pd-Catalyzed Cross-Coupling
Shohei Nishitani,† Ryo Sekiya,† Ikuya Matsumoto, and Takeharu Haino,* Chem.
Lett., 2021, 50, 664–667.
DOI:10.1246/cl.200844
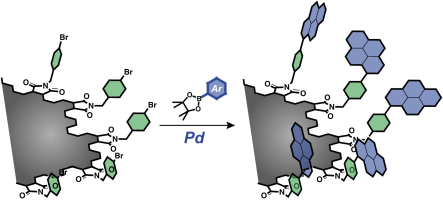
Abstract: Top-down methods produce nanographenes with many carboxy groups on their edges. These functional groups can be utilized for developing multichromophoric systems. As proof of concept, pyrene is installed on the edges by Pd-catalyzed cross-coupling reactions. The lack of monomer emissions from the functionalized nanographenes indicates that the neighboring chromophores are sufficiently distant to form the excimer. The pyrene-installed nanographene emits bluish-white-light. Its lipophilic nature allows fabricating a nanographene-dispersed polymethyl methacrylate film emitting visible light.
(65) Edge-Functionalized Nanographenes (Review Article)
Ryo Sekiya* and Takeharu Haino,* Chem. Eur. J., 2021, 27, 187–199 (Review Showcase, Outstanding Review-type Article).
DOI:10.1002/chem.202003370

Abstract: Nanographenes (NGs) have recently emerged as new carbon materials. Their nanoscale size results in a size-dependent quantum confinement effect, opening the band gap by a few eV. This energy gap allows NGs to be applied as optical materials. This property has attracted researchers across multiple scientific fields. The photophysical properties of NGs can be manipulated by introducing organic groups onto their basal planes and/or into their edges. In addition, the integration of organic functional groups into NGs results in NG-based hybrid materials. These features make the post-synthetic modification of NGs an active research area. As obtainable information on chemically functionalized NGs is limited owing to their nonstoichiometry and structural uncertainty, their structural characterization requires a combination of multiple spectroscopic methods. Therefore, information on the characterization procedures of recently published chemically functionalized NGs is of value for advancing the field of NG-based hybrid materials. The present review focuses on the structural characterization of chemically functionalized NGs. It is hoped that this review will help to advance this field
(64) Programmed Dynamic Covalent Chemistry System of Addition-Condensation
Reaction of Phenols and Aldehydes
Hiroto Kudo,* Daisuke Shimoyama, Ryo Sekiya, and Takeharu Haino, Chem.
Lett., 2021, 50, 825–831.
DOI:0.1246/cl.200773
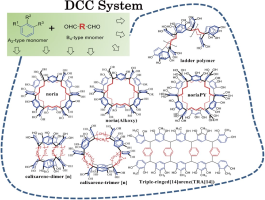
Abstract: Application of a dynamic covalent chemistry strategy to the reversible reaction system of resorcinol and α, ω-alkanedials [(CH2)m(CHO)2] (m = 2–10) in ethanol in the presence of hydrogen chloride (HCl) solution as a catalyst at 80 °C for 48 h afforded the thermodynamically most stable products with high selectivity. The reaction of 1,4-butanedial afforded a ladder polymer containing calixarene skeletons in the main chain in quantitative yield. 1,5-Pentanedial gave Noria, a water-wheel-like cyclic oligomer, in high yield. Calixarene-dimer-type cyclic oligomers were formed selectively from 1,6-hexanedial, 1,8-octanedial, 1,10-decanedial, and 1,12-dodecanedial, while calixarene-trimer-type cyclic oligomers were obtained selectively from 1,7-heptanedial, 1,9-nonanedial, and 1,11-undecanedial. The Noria like macrocyles NoriaPY NoriaMP and NoriaEP could be also synthesized via DCC using pyrogallol, 3-methoxyphenol, and 3-ethoxyphenol. A triple-ringed[14]arene could be synthesized via DCC using the reaction of 2-methylresorcinol and m-benzenedicarbaldehyde.
(63) Absorption of Chemicals in Amorphous Trisresorcinarene
Daisuke Shimoyama,† Ryo Sekiya,† and Takeharu Haino,* Chem. Commun., 2020,
56, 12582-12585.
DOI:10.1039/D0CC05066K
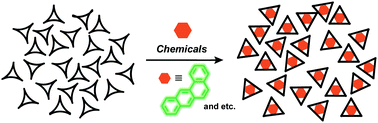
Abstract:Trisresorcinarene is an interesting class of macrocyclic host. Its insoluble nature and conformationally flexible structure allow its application as a solid absorbent. Various aromatic and aliphatic hydrocarbons are absorbed into the amorphous solid of the trisresorcinarene, demonstrating the versatile absorption capability of trisresorcinarene.
(62) Upper-Rim Functionalization and Supramolecular Polymerization of a
Feet-to-Feet-Connected Biscavitand
Daisuke Shimoyama, Ryo Sekiya, and Takeharu Haino,* Chem. Commun., 2020,
56, 3733-3736.
DOI:10.1039/D0CC00933D
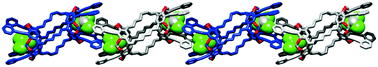
Abstract: An octaiodobiscavitand was synthesized via an aromatic Finkelstein iodination reaction in good yield. Sonogashira and Suzuki coupling reactions of the octaiodobiscavitand gave rise to upper-rim-functionalized biscavitands that self-assembled to form a supramolecular polymer in the solid state.
(61) One-Dimensional Arrangement of NORIA in the Solid-State
Daisuke Shimoyama,† Ryo Sekiya,† Hiroto Kurdo, and Takeharu Haino,* CrystEngComn,
2020, 22, 4740-4747.
DOI:10.1039/D0CE00650E
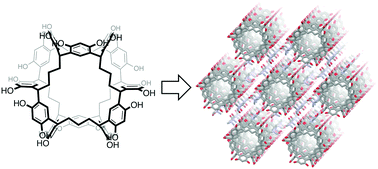
Abstract: NORIA is a synthetic macrocycle consisting of twelve resorcinol rings. The hydroxy groups surrounding the surface of NORIA make it difficult for NORIA to be dissolved in organic solvents, except for polar solvents, a property which is ideal for developing solid-state materials. For developing solid-state materials, information about the effect of the crystallization conditions on the crystal packing of NORIA is required, although only limited examples have been reported so far. We find that the crystal packing of NORIA depends heavily on the crystallization conditions. Single crystals are grown in DMF solutions by the slow diffusion of pentane, cyclohexane, methylcyclohexane, benzene, and toluene into the solution. X-ray diffraction analysis demonstrates that the saturated hydrocarbon vapors induce the molecules to organize into a triclinic crystal packing, whereas another crystal packing, wherein NORIA is arranged linearly in the hexagonal crystal, is formed in the case of aromatic vapors. In the five crystals, DMF molecules are trapped by NORIA and interact with neighboring hosts through hydrogen bonding, indicating that the DMF molecules function as linkers. The X-ray crystal structures suggest that the planarity of the guests is a factor behind the formation of the hexagonal crystal packing. Electron density maps and the solvent accessible surface of NORIA suggest that small molecules, such as water, can access the cavity. The large solvent-accessible volumes in the unit cells of both crystal packings demonstrate that NORIA functions as an excellent building block for lattice inclusion compounds.
(60) A Regulable Internal Cavity inside a Resorcinarene-based Hemi-Carcerand
Kentaro Harada,† Ryo Sekiya,† and Takeharu Haino,* Chem. Eur. J., 2020,
26, 5810-5817(Cover Picture).
DOI:10.1002/chem.201905805
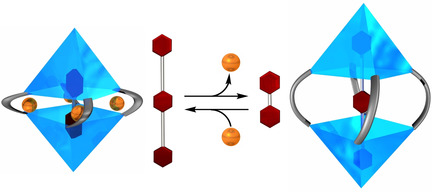
Abstract: Covalent organic capsules, such as carcerands and hemicarcerands, are an interesting class of molecular hosts. These container molecules have confined spaces capable of hosting small molecules, although the fact that the size of the inner cavities cannot be changed substantially limits the scope of their applications. The title covalently linked container was produced by metal-directed dimerization of a resorcinarene-based cavitand having four 2,2′-bipyridyl arms on the wide rim followed by olefin metathesis at the vertices of the resulting capsule with a second-generation Grubbs catalyst. The covalently linked bipyridyl arms permit expansion of the inner cavity by demetalation. This structural change influences the molecular recognition properties; the metal-coordinated capsule recognizes only 4,4′-diacetoxybiphenyl, whereas the metal-free counterpart can encapsulate not only 4,4′-diacetoxybiphenyl, but also 2,5-disubstituted-1,4-bis(4-acetoxyphenylethynyl)benzene, which is 9.4 Å longer than the former guest. Molecular mechanics calculations predict that the capsule expands the internal cavity to encapsulate the long guest by unfolding the folded conformation of the alkyl chains, which demonstrates the flexible and regulable nature of the cavity. Guest competition experiments show that the preferred guest can be switched by metalation and demetalation. This external-stimuli-responsive guest exchange can be utilized for the development of functional supramolecular systems controlling the uptake, transport, and release of chemicals.
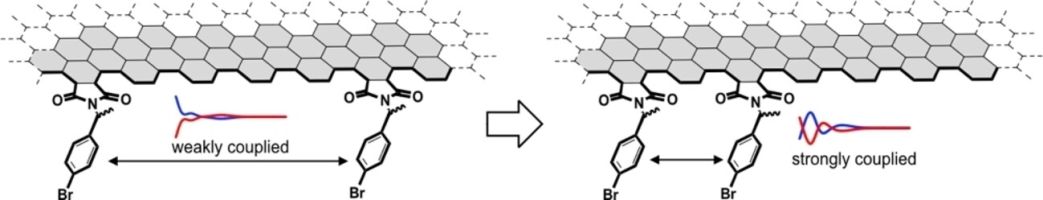
(59) Chirality-Embedded Nanographene
Shohei Nishitani,† Ryo Sekiya,† and Takeharu Haino,* Angew. Chem. Int.
Ed., 2020, 59, 669-673(Hot Paper).
DOI:10.1002/anie.201910040
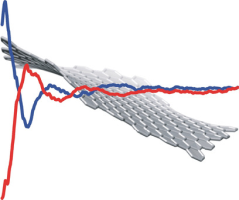
Abstract:The development of chiral nanographenes has mostly been carried out by bottom-up methods and examples of species developed by the post-modification of nanographenes prepared by top-down methods remain limited. We show that the attachment of chiral functional groups onto the edge of nanographenes generates chirality on the surface. X-ray diffraction analysis and DFT calculations indicate that the chirality of the functional groups is transferred to the surface via steric interactions from the chiral center through the five-membered cyclic imide to the nanographene edge. The exciton coupling between the p-bromophenyl groups confirms that the functional groups are arranged on the armchair edges at distances that permit exciton coupling, which provides information about their relative orientation. These pieces of information help to elucidate the edge structure of nanographenes prepared by top-down methods.
(58) Feet-to-Feet Connected Trisresorcinarenes
Daisuke Shimoyama, Ryo Sekiya, Hiroto Kudo, and Takeharu Haino,* Org. Lett.,
2020, 22, 352-356 (Cover Picture).
DOI:10.1021/acs.orglett.9b03693
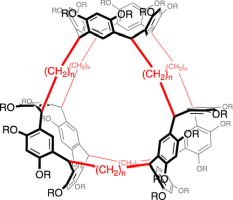
Abstract: The macrocyclization of resorcinol and odd-numbered bisdioxolanes under acidic conditions produced feet-to-feet connected trisresorcinarenes possessing three resorcinarene units linked with odd-numbered alkyl chains. The formation of trisresorcinarenes was confirmed using high-resolution mass spectrometry and NMR spectroscopy. The trisresorcinarenes were isolated as protected forms in moderate yields. The protected trisresorcinarenes exhibited D3h symmetry in conformation in solution. Crystal structure analysis revealed that the protected trisresorcinarenes possess large inner spaces surrounded by three resorcinarene units.
(57) Chemically Functionalized Two Dimensional Carbon Materials (Review
Article)
Ryo Sekiya* and Takeharu Haino,* Chem. Asian J., 2020, 15, 2316-2328 (Special
Issue: The Chemistry of 2D Materials Membranes).
DOI:/10.1002/asia.202000196
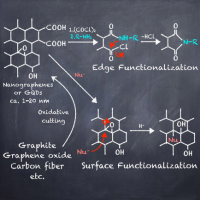
Abstract: Nanographenes (NGs), also known as graphene quantum dots, have recently been developed as nanoscale graphene fragments. These nanocarbon species can be excited with UV light and emit light from the UV-to-visible region. This photoemission has received great attraction across multiple scientific fields. NGs can be produced by cutting off carbon sources or fusing small organic molecules to grow graphitic structures. Furthermore, the organic synthesis of NGs has been intensely studied. Recently, the number of research papers on postsynthetic modification of NGs has gradually increased. Installed organic groups can tune the properties of NGs and provide new functionalities, opening the door for the development of sophisticated carbon-based functional materials. This review sheds light on recent progress in the postsynthetic modification of NGs and provides a brief summary of their production methods.
(56) A Protocol for Separation of Nanographenes
Ikuya Matsumoto,† Ryo Sekiya,† and Takeharu Haino,* RSC Adv., 2019, 9,
33843-33846(Hot Article).
DOI:10.1039/C9RA07528C

Abstract:Top-down methods are convenient preparative methods for nanographenes, although the products consist of graphene fragments with a broad size distribution. We show that a combination of dialysis membranes (50, 25, 15, 8, and 2 kD) can conveniently separate nanographenes into five size distributions. The separated nanographenes can be employed as starting materials for carbon-based functional materials.
(55) Intrinsic Emission from Nanographenes
Kairi Yamato,† Ryo Sekiya,† Shohei Nishitani, and Takeharu Haino,* Chem.
Asian. J., 2019, 14, 3213-3220.
DOI:10.1002/asia.201900906
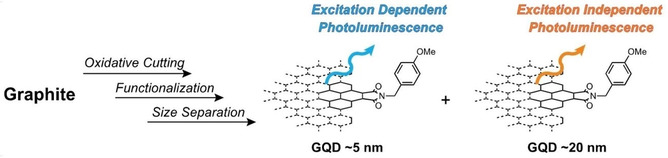
Abstract: Top-down approaches have been widely used as convenient methods for the production of nanographenes. To understand the photoemission properties of nanographenes, their separation and the optical properties of the individual fractions is important. By using a combination of size-exclusion and silica-gel-adsorption chromatography, we separated lipophilic nanographenes that contained para-methoxybenzyl groups. The mixture consisted of large (average 19.8 nm) and small (average 4.9 nm) nanographenes, whilst unreacted carboxy groups remained in the latter group. Optical measurements revealed that oxygen-containing functional groups had little influence on the photoemission of the nanographenes, thus indicating that the intrinsic emission, that is, emission from the sp2 surfaces, was responsible for the photoemission. Two photoemission bands were observed for all of the fractions, which likely originated from the edge and inner parts of nanographene.
(54) Near-infrared Emitting Nitrogen-Doped Nanographenes
Kairi Yamato,† Ryo Sekiya,† Kaho Suzuki,† and Takeharu Haino,* Angew. Chem.
Int. Ed., 2019, 58, 9022-9026.
DOI:10.1002/anie.201901510
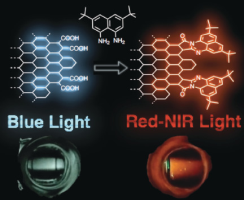
Abstract: The quantum-size effect, which enables nanographenes to emit photoluminescence (PL) in the UV to visible region, has inspired intense research. However, the control of the PL properties of nanographenes through manipulation of their π-system by post-modifications is not well developed. By utilizing a ring-closure reaction between an aromatic 1,2-dicarboxylic acid and a 1,8-naphthalenediamine derivative, which produces a perimidine framework, nitrogen-doped nanographenes were realized. Two nanographenes produced by a one-pot reaction of edge-oxidized nanographene (GQD-2) with 1,8-naphthalenediamine derivatives (GQD-1 a and GQD-1 b) displayed an absorption band extending to >1000 nm; furthermore, the PL wavelength of GQD-1 a was significantly red-shifted into the near-infrared (NIR) region in which it can be used for bioimaging. Time-dependent DFT calculations of model nanographenes showed that the functional groups narrow the HOMO–LUMO gap, realizing the NIR-emitting nanographenes.
(53) Substituent-Controlled Racemization of Dissymmetric Coordination Capsules
Kentato Harada,† Ryo Sekiya,† Takeshi Maehara,† and Takeharu Haino,* Org.
Biomol. Chem., 2019, 17, 4729-4735 (Cover Picture).
DOI:10.1039/C9OB00388F
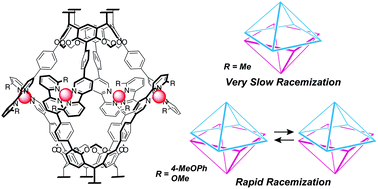
Abstract:We report the effect of substituents (methyl, isopropyl, methoxy, and methoxyphenyl) at the 6′-position of the 2,2′-bipyridyl arms on the racemization of dissymmetric coordination capsules 1a–d. When the capsules included (R)-4,4′-diacetoxy-2,2′-benzyloxycarboxyl-biphenyl ((R)-3), the (M)-helical conformer was enriched with a diastereomeric excess (de%) of >98% for 1a, 31% for 1b, 81% for 1c and 75% for 1d. The entrapped guests in 1a, 1c and 1d can be removed by washing the solid containing the host–guest complexes with diethyl ether. The rate of racemization in THF follows the order of 1c > 1d ≫ 1a. X-ray crystal structural analysis and density functional theory calculation of model complex 4c indicate a distorted tetrahedral coordination of the Cu(I) center, and UV-vis absorption spectroscopy indicates similar coordination environments in 1c and 4c. A series of experiments demonstrates that the racemization rate depends on the dihedral angles of the bipyridyl arms, and the angles are regulated by the substituents. The methoxy and methoxyphenyl substituents in 1c and 1d enlarge the dihedral angles of the bipyridyl arms. This facilitates the access of solvent molecules to the Cu(I) centers and promotes racemization. The slower racemization of 1d can be ascribed to the steric protection of the Cu(I) centers from incoming solvent molecules by the p-methoxyphenyl group.
(52) Separation of Spectroscopically Uniform Nanographenes
Kairi Yamato,† Ryo Sekiya,† Manabu Abe, and Takeharu Haino,* Chem. Asian
J., 2019, 14, 1786-1791 (Cover Picture).
DOI:10.1002/asia.201801632
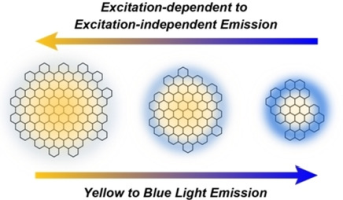
Abstract: Excitation-dependent photoluminescence (PL) is a well-known property of graphene quantum dots (GQDs). For the development of carbon-based photofunctional materials, GQDs possessing uniform PL properties are in high demand. A protocol has been established to separate spectroscopically uniform lipophilic GQD-1 a from a mixture of GQD-1 mainly composed of GQD-1 a and GQD-1 b. The mixture of GQD-1 was synthesized through the reaction of p-methoxybenzylamine with GQD-2 prepared from graphite by common oxidative exfoliation. Size-exclusion chromatography gave rise to GQD-1 a and GQD-1 b, with diameters of 19.8 and 4.9 nm, respectively. Large GQD-1 a showed that the PL was fairly independent of the excitation wavelengths, whereas the PL of small GQD-1 b was dependent on excitation. The excitation-dependent nature is most likely to be associated with the structures of sp2 domains on the graphene surfaces. The large sp2-conjugated surface of GQD-1 a is likely to possess well-developed and large sp2 domains, the band gaps of which do not significantly vary. The small sp2-conjugated surface of GQD-1 b produces small sp2-conjugated domains that generate band gaps differing with domain sizes.
(51) Organogelators of 5,17-Difunctionalized Calix[4]arene with Pinched
Cone Conformation
Lai Nang Duy,† Ryo Sekiya,† Masatoshi Tosaka, Shigeru Yamago, Takashi Nishino,
Takayuki Ichikawa, and Takeharu Haino,* Chem. Lett., 2019, 48, 43–46.
DOI:10.1246/cl.180819
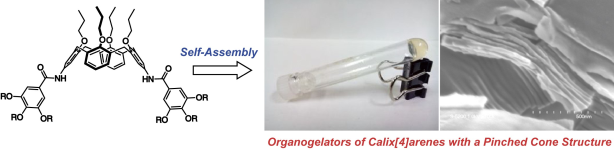
Abstract:5,17-Difunctionalized calix[4]arenes 1a,b were found to function as low molecular-weight organic gelators. They formed organogels with a variety of organic solvents. SEM images showed 1a formed stacked structures in the xerogels. Single-crystal X-ray analysis of 1c as well as AFM, XRD, and SAXS measurements of 1a indicated that the xerogels contained lamellar structures of the calix[4]arenes.
(50) Tunable Confined Space inside Coordination Capsules
Takeshi Maehara,† Ryo Sekiya,† Kentaro Harada, and Takeharu Haino,* Org.
Chem. Frontier., 2019, 6, 1561-1566 (Cover Picture).
DOI:10.1039/C9QO00010K
From the themed collection: In celebration of Julius Rebek’s 75th Birthday
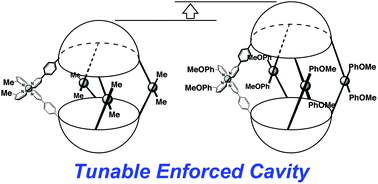
Abstract:Controlling and tuning the molecular recognition properties is a crucial task in host–guest chemistry. The size and dimension of the guest-binding space inside self-assembled capsules 1a–c is successfully determined by installing the substituents at the 6′-position of 2,2′-bipyridyl arms. X-ray diffraction analysis and DFT calculations at the M06-2X/6-31G(d,p)+LanL2DZ level of theory demonstrate that the p-methoxyphenyl group expands the dihedral angle of the 2,2′-bipyridyl arms through its π-stacking interaction to the 2,2′-bipyridyl arm, whereas the steric interaction of the isopropyl group to the neighboring bipyridyl arm slightly reduces the dihedral angle of the two bipyridyl groups. The Cu(I)-united 2,2′-bipyridyl arms function as a hinge; accordingly, installing the p-methoxyphenyl group extends the cavity by ca. 2 Å, whereas the isopropyl group shrinks the cavity more than that of the capsule 1a with methyl groups at the 6′-positions. These steric interactions influence the molecular recognition of the capsules 1a–c for rigid ditopic guest 2a as well as flexible ones 2b–f. The guest selectivity inside the enforced cavity is determined by varying the substituents.
(49) Facile Synthesis of an Eight-Armed Star-Shaped Polymer via Coordination-Driven
Self-Assembly of a Four-Armed Cavitand
Natsumi Nitta, Mei Takatsuka ,Shinichi Kihara, Ryo Sekiya, and Takeharu
Haino,* ACS macro Lett., 2018, 7, 1308–1311(Cover Picture).
DOI:10.1021/acsmacrolett.8b00669
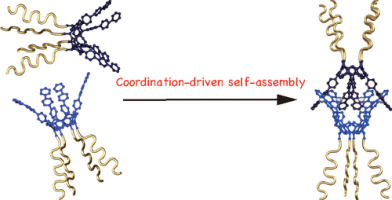
Abstract:The polystyrene chains were installed at the lower rim of a resorcinarene-based cavitand via reversible addition–fragmentation (RAFT) polymerization to form a four-armed star-shaped polymer. A star-shaped polystyrene-functionalized supramolecular capsule was prepared through the coordination-driven self-assembly of the four-armed start-shaped polymer with silver ions. The eight-armed start-shaped supramolecular capsule encapsulated 4,4′-diacetoxybiphenyl as did a cavitand-based self-assembled capsule. A DOSY measurement indicated that the eight-armed star-shaped polymer was twice as large as the four-armed star-shaped polymer. The solution behaviors of these compounds resulted in a difference in their zero-shear viscosities.
(48) Pseudorotaxanes in the Gas Phase: Structure and Energetics of Protonated
Dibenzylamine-Crown Ether Complexes
Motoki Kida, Daisuke Shimoyama, Toshiaki Ikeda, Ryo Sekiya, Tahekaru Haino,
Takayuki Ebata, Christophe Jouvet and Yoshiya Inokuchi,* Phys. Chem. Chem.
Phys., 2018, 20, 18678–18687.
DOI:10.1039/C8CP02707B
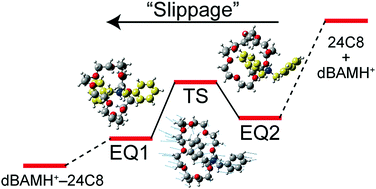
Abstract:We observe UV spectra of protonated dibenzylamine (dBAMH+) and its complexes with 15-crown-5 (dBAMH+–15C5), 18-crown-6 (dBAMH+–18C6), and 24-crown-8 (dBAMH+–24C8) under cold (∼10 K) gas-phase conditions by UV photodissociation (UVPD) and UV–UV hole-burning (HB) spectroscopy. The UVPD spectrum of the dBAMH+–15C5 complex shows an extensive low-frequency progression, which originates from a unique conformation of the dBAMH+ part with benzene rings facing closely to each other, while UVPD and calculation results suggest open conformations of the dBAMH+ part for dBAMH+–18C6 and dBAMH+–24C8. UV–UV HB spectra of the dBAMH+–24C8 complex indicate that there exist at least two conformers; multiple conformations can contribute to high stability of dBAMH+–24C8 pseudorotaxane due to “conformational” entropic effects. The UVPD experiment indicates that the dissociation probability of dBAMH+–24C8 into dBAMH+ and 24C8 is substantially smaller than that of dBAMH+–15C5 and dBAMH+–18C6, which can be related to the barrier height in the dissociation process. The energetics of the dBAMH+–24C8 complex is investigated experimentally with NMR spectroscopy and theoretically with the global reaction route mapping (GRRM) method. An energy barrier of ∼60 kJ mol−1 is present in the pseudorotaxane formation in solution, whereas there is no barrier in the gas phase. In the course of the photodissociation, excited dBAMH+–24C8 complexes can be trapped at many local minima corresponding to multiple conformations. This can result in effective dissipation of internal energy into degrees of freedom not correlated to the dissociation and decrease the dissociation probability for the dBAMH+–24C8 complex in the gas phase. The energy barrier for the pseudorotaxane formation in solution originates not simply from the slippage process but rather from solvent effects on the dBAMH+–24C8 complex.
(47) Majority-Rules Effect and Allostery in Molecular Recognition of Calix[4]arene-Based
Triple-Strand
Yutaro Yamasaki, Hidemi Shio, Tomoko Amimoto, Ryo Sekiya, and Takeharu
Haino,* Chem. Eur. J., 2018, 24, 8558-8568(Frontispiece).
DOI:10.1002/chem.201800997
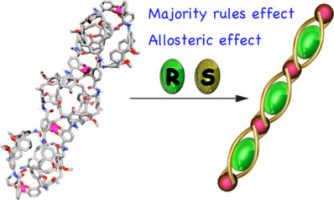
Abstract:The triple-stranded metallohelicates 1 a,/1 b–3 a/3 b possessing internal guest binding cavities surrounded by calix[4]arene units were synthesized through coordination-driven self-assembly. UV/Vis titration experiments verified that the metallohelicates encapsulated N-methyl pyridinium cations bearing amino acid groups to form host–guest complexes. The guest chirality was transferred to the helicity of the helicates through the steric contact between the stereogenic center of the amino acid group and the metal cores. The (M) helicity was induced when guests the (R)-4--(R)-6 were accommodated within the cavities. The multiple guest complexation within the self-assembled helicates 2 a and 3 a displayed large positive cooperative effects, indicating that the first guest complexation preorganizes the rest of the cavities to facilitate a subsequent guest binding. This cooperativity results in the majority-rules effect in the chiral guest binding for 2 a and 3 a.
(46) A Supramolecular Polymer Network of Graphene Quantum Dots
Yuichiro Uemura, Kairi Yamato, Ryo Sekiya, and Takeharu Haino,* Angew.
Chem. Int. Ed., 2018, 57, 4960-4964.
DOI:10.1002/anie.201713299
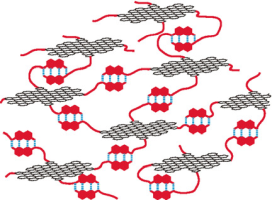
Abstract:Graphene quantum dot (GQD)–organic hybrid compounds (GQD-2 b–e) were prepared by introducing 3,4,5-tri(hexadecyloxy)benzyl groups (C16) and linear chains terminated with a 2-ureido-4-[1H]-pyrimidinone (UPy) moiety onto the periphery of GQD-1. GQD-2 b–e formed supramolecular assemblies through hydrogen bonding between the UPy units. GPC analysis showed that GQDs with high loadings of the UPy group formed larger assemblies, and this trend was confirmed by DOSY and viscosity measurements. AFM images showed the polymeric network structures of GQD-2 e on mica with flat structures (ca. 1.1 nm in height), but no such structures were observed in GQD-2 a, which only carries the C16 group. GQD-2 c and GQD-2 d formed organogels in n-decanol, and the gelation properties can be altered by replacing the alkyl chains in the UPy group with ethylene glycol chains (GQD-3). GQD can thus be used as a platform for supramolecular polymers and organogelators by suitable chemical functionalization.
(45) Synthesis and Dimerization Studies of a Lipophilic Photoresponsive
Aryl-Extended Tetraurea-calix[4]pyrrole
Ryo Sekiya, Alejandro Díaz-Moscoso, and Pablo Ballester,* Chem. Eur. J.,
2018, 24, 2182-2191.
DOI:10.1002/chem.201704777
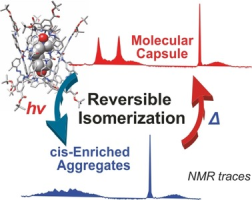
Abstract:We describe the syntheses of the lipophilic aryl-extended α,α,α,α-tetraurea-phenyl-calix[4]pyrrole 1, featuring four appended azo-phenyl groups with two tert-butoxy carbonyl meta-substituents and its photo-inactive counterpart 2. In CD2Cl2 solutions, both tetraurea-calix[4]pyrroles self-assemble into dimeric capsules by encapsulating one molecule of a suitable bis-N-oxide or two molecules of a mono-N-oxide. The dimeric capsules are mainly stabilized by a cyclic array of sixteen hydrogen bonds established between the eight unidirectionally oriented urea groups. Photoirradiation experiments demonstrated the trans-to-cis isomerization of the azo-phenyl groups and the formation of a plethora of stereo isomeric cis-azo-enriched capsular assemblies. The highly cis-azo enriched capsular assemblies seem to show a reduced stability and their involvement in equilibria with non-capsular counterparts that also bind the N-oxides. The thermally induced cis-to-trans interconversion processes demonstrated the reversibility of the photoisomerization and the photostability of most binding partners. An equimolar mixture of the two tetraureas produced two homodimeric capsules and the heterodimeric counterpart in a ratio close to statistical distribution.
(44) Synthesis and Structure of Feet-to-Feet Connected Bisresorcinarenes
Daisuke Shimoyama, Toshiaki Ikeda, Ryo Sekiya, and Takeharu Haino,* J.
Org. Chem., 2017, 82, 13220-13230.
DOI:10.1021/acs.joc.7b02301
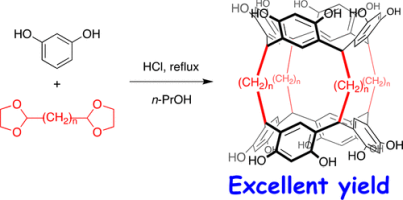
Abstract:Bisresorcinarenes 1a–d were obtained in excellent yields, and 1e was finally obtained in 50% yield. X-ray diffraction analysis showed that 1a and 1b adopted helical conformations, whereas the two resorcinarenes of 1c–e were in parallel orientations in which the clefts of the aliphatic chains entrapped one or two solvent molecules. The conformational study revealed that the helix interconversion between the (P)- and (M)-helical conformers depended on the length of the aliphatic chains. 1a had the largest energetic barrier to helix interconversion, while in 1b, its more flexible aliphatic chains lowered its energetic barriers. The P/M interconversion of 1a was coupled with the clockwise/anticlockwise interconversion of the interannular hydrogen bonding of the two resorcinarenes. The large negative entropic contributions indicate that the transition state is most likely more ordered than the ground states, suggesting that the transition state is most likely symmetric and is solvated by water molecules. Calculations at the M06-2X/6-31G(d,p) level revealed that the more stable (P)-conformation has clockwise interannular hydrogen bonding between the two resorcinarenes.
(43) Hexameric Assembly of 5,17-Di-substituted Calix[4]arene in the solid
state
Yutaro Yamasaki, Ryo Sekiya, and Takeharu Haino,* CrystEngComm, 2017, 19,
6744-6751.
DOI:10.1039/C7CE01515A
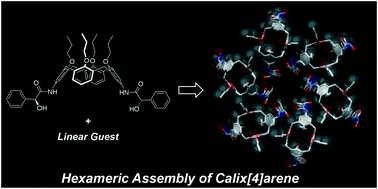
Abstract: Chiral 5,17-difunctionalized-25,26,27,28-tetrapropyloxycalix[4]arene possessing (S)-mandelamide arms ((S,S)-1) afforded cocrystals (S,S)-1·(solvent) (solvent = MeOH, EtOH, 1-PrOH, 2-PrOH, and CH3CN). X-ray diffraction analysis revealed that (S,S)-1 formed head-to-tail columnar structures. In most cocrystals (MeOH, EtOH, 1-PrOH, and CH3CN), the columnar structures were arranged hexamerically to form a chiral hexagonal channel, which is a rare example of calix[4]arene derivatives in the solid state. The fact that the branched guest (2-PrOH) and the racemic mixture of the host formed different crystal packings wherein no hexameric structure existed indicates that both the linearity of the guest and the homochirality of the host are important factors for driving the columnar structures to form the hexameric assembly. Apohost (S,S)-1apo showed interesting adsorption behavior; it adsorbed benzene among the organic molecules tested. The adsorption and desorption processes were repeated several times, demonstrating the sponge-like character of (S,S)-1apo.
(42) Photoluminescence Response of Graphene Quantum Dots toward Organic
Bases and an Acid
Kaho Suzuki, Ryo Sekiya, and Takeharu Haino,* Photochem. PhotoBio. Sci.,
2017, 16, 623-626.
DOI:10.1039/C7PP00067G
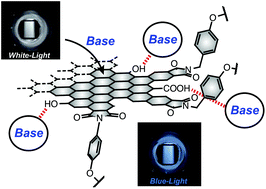
Abstract:Edge-modified graphene quantum dots (GQD-1) showed base-dependent photoluminescence (PL) responses. DBN, DBU, and Et3N gradually enhanced the PL intensity and then caused quenching, whereas quenching was not observed when pyridine and pyrimidine were used. The quenching can be explained by the photoinduced electron transfer from the anchored bases at the periphery to the emissive sites of GQD-1.
(41) Site-Selective Encapsulation of Different Anions in a Quadruply Interlocked
Dimer
Ryo Sekiya,* Morihiko Fukuda, and Reiko Kuroda,* Org. Biomol. Chem., 2017,
15, 4428-4235.
DOI:10.1039/C7OB00328E
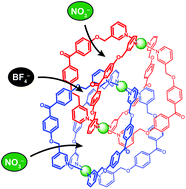
Abstract:Interlocked dimer 2, which is composed of two physically interlocked monomers 1, has three cavities (cavity A × 2 and cavity B × 1) and can encapsulate three anions, such as NO3− and BF4−, one anion per cavity. There are six possible encapsulation patterns, A–F; two (A and F) contain only one kind of anion and the others (B–E) contain both NO3− and BF4− at the same time with different ratios and with different positions. Anion competition experiments showed that in addition to F, which encapsulates three NO3− ions, C, in which NO3− and BF4− ions are captured in cavities A and cavity B, respectively, was selectively formed. Detailed investigations have revealed that B–E were formed by dimerization, but three of the four were subjected to anion exchange and converged into C or F. This selective formation can be explained by the fact that NO3− is a better anion template than BF4−, as well as the molecular structure of the interlocked dimer; cavities A are surrounded by four bridging ligands and can be accessed by free anions, whereas no space available for anion exchange is present around cavity B because this cavity is surrounded by eight bridging ligands. Therefore, the BF4− ions in cavities A are expelled by free NO3−, but the BF4− ion in cavity B is not, resulting in the selection of C and F. We have found that the volume of the cavity influenced anion recognition. New interlocked dimer 3, which has smaller cavities than those of 2, captured three NO3− ions to form F, whereas only a small amount of an interlocked dimer that contains both NO3− and BF4− was formed.
(40) Supramolecular Graft Copolymerization of a Polyester via Guest-Selective
Encapsulation of a Self-Assembled Capsule
Yuta Tsunoda, Mei Takatsuka, Ryo Sekiya, and Takeharu Haino,* Angew. Chem.
Int. Ed., 2017, 56, 2613-2618.
DOI:10.1002/anie.201611394
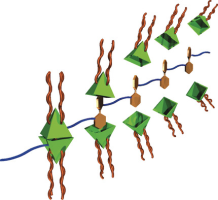
Abstract:Repeating guest units of polyesters poly-(R)-2 were selectively encapsulated by capsule 1(BF4)4 to produce supramolecular graft polymers. The encapsulation of the guest units was confirmed by 1H NMR spectroscopy. The graft polymer structures were confirmed by the increase in the hydrodynamic radii and the solution viscosities of the polyesters upon complexation of the capsule. After the capsule was formed, atomic force microscopy showed extension of the polyester chains. The introduction of the graft chains onto poly-(R)-2 resulted in the main chain of the polymer having an M-helical morphology. The complexation of copolymers poly-[(R)-2-co-(S)-2] by the capsule gave rise to the unique chiral amplification known as the majority-rules effect.
(39) Vanadium(V) complexes of some bidentate hydrazone ligands and their
bromoperoxidase activity
Piyali Adak, Bipinbihari Ghosh, Bholanath Pakhira, Ryo Sekiya, and Reiko
Kuroda, Shyamal Kumar Chattopadhyay,* Polyhedron, 2017, 127, 135-143.
DOI:10.1016/j.poly.2017.01.054
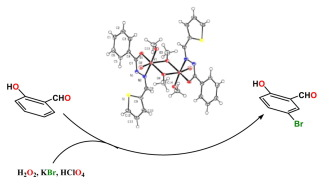
Abstract:Dinuclear methoxy bridged complexes of vanadium, [VO(µ-OMe)(OMe)(L)]2 (1–3) have been synthesized from the reaction of VOSO4·H2O with triethylamine and the respective hydrazone ligand. The compounds have been characterized by spectroscopic methods and determination of single crystal X-ray structure of one of them (1). DFT and TD-DFT calculations were used to understand the electronic structures of the complexes and their electronic spectra respectively. Though the dimeric complexes are stable in the solid state, the ESI-MS spectra as well as 1H NMR spectra of the complexes suggest that in solution the monomeric forms of the complexes are the major species. The V(V) complexes in DMF were used to catalyze the oxidative bromination of salicylaldehyde, in aqueous H2O2/KBr in the presence of HClO4 at room temperature. The complexes show exceptionally high bromoperoxidase activity with salicylaldehyde as a model substrate to produce 5-bromo salicylaldehyde in good yield and high TOF and TON. Therefore, these complexes behave as functional models of vanadate dependent bromoperoxidase enzyme.
(38) Cooperative self-assembly of carbazole driven by multple dipole-dipole
interactions
Toshiaki Ikeda, Tatsuya, Iijima, Ryo Sekiya, Osamu, Takahashi, and Takeharu
Haino,* J. Org. Chem., 2016, 81, 6832-6837.
DOI:10.1021/acs.joc.6b01169

Abstract:Carbazole possessing phenylisoxazoles self-assembled in a cooperative manner in decalin. X-ray crystal structure analysis revealed that the isoxazole dipoles align in a head-to-tail fashion. DFT calculations suggested that the linear array of dipoles induced the polarization of each dipole, leading to an increase in dipole–dipole interactions. This dipole polarization resulted in cooperative assembly.
(37) Allostery in Guest Binding of Rim-to-Rim Connected Homptopic Biscavirand
Daisuke Shimoyama, Htomi Yamada, Toshiaki Ikeda, Ryo Sekiya, and Takeharu
Haino,* Eur. J. Org. Chem., 2016, 3300-3303 (Cover Picture).
DOI:10.1002/ejoc.201600410
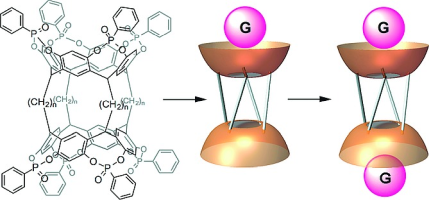
Abstract:Rim-to-rim-connected phosphonate biscavitands 1a and 1b were synthesized. Crystal structure analysis and variable-temperature NMR spectroscopy studies reveal that 1a is more rigid in conformation than 1b. Biscavitands 1a and 1b bind cationic guests G1–G3 through hydrogen bonding and CH–π interactions, demonstrating positive and negative allosteric effects, respectively. The allosteric effects in guest binding are associated with the conformational flexibilities of 1a and 1b. The positive allosteric effect of 1a is directed by the tightly connected cavities, in which information of the first guest binding is transferred to the remaining cavity, which becomes preorganized. In contrast, a slightly negative allosteric effect is found in more-flexible 1b.
(36) Frozen Dissymmetric Cavity in Resorcinarene-Based Coordination Capsule
Taisuke Imamura, Takeshi Maehara, Ryo Sekiya, and Takeharu Haino,* Chem.
Eur. J., 2016, 22, 3250-3254.
DOI:10.1002/chem.201505183

Abstract:By introducing slight structural modifications to a D4-symmetric coordination capsule, we succeeded in isolating the nearly enantiopure capsules (P)- and (M)-2 a(BF4)4. Chiral guest, dibenzyl 4,4′-diacetoxy-6,6′-dimethyl-[1,1′-biphenyl]-2,2′-dicarboxylate (3) was encapsulated within the dissymmetric cavity of 2 a(BF4)4, resulting in a high diastereoselectivity of >99 % de. The encapsulated guest was successfully removed from the complex without racemization through precipitation of the empty capsule. CD spectra confirmed that the chirality of the capsule was maintained in THF and 1,4-dioxane for long periods, whereas a small amount of acetonitrile accelerated racemization of the empty capsule. The activation parameters of the racemization reaction were determined in dichloromethane and 1,2-dichloroethane, resulting in positive enthalpic contributions and large negative entropic contributions, respectively. Accordingly, the racemization fits a first-order kinetic model. Mechanically coupled Cu+-2,2′-bipyridine coordination centers were responsible for the high-energy barrier of racemization and led to the unique chiral memory of the dissymmetric cavity, which was turned off by the addition of acetonitrile.
(35) Hydrogen-Bonded Hexameric Cluster of Benzyl Alcohol in the Solid State
Polymeric Organization of p-tert-Butylcalix[5]arene
Yasunori Kajiki, Ryo Sekiya, and Takeharu Haino,* Supramol. Chem., 2016,
28, 444-449.
DOI:10.1080/10610278.2015.1117084
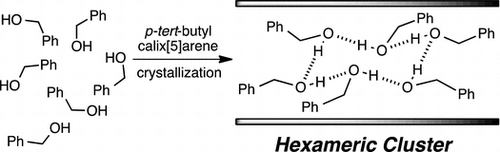
Abstract:An uncommon hydrogen-bonded hexameric cluster of benzyl alcohol (2) was formed in hydrophobic space in the layered organisation of the one-dimensional polymeric zigzag array of 1 in the solid state. The hexameric cluster adopted a distorted cyclohexane-like (12) O–H⋯O hydrogen bond network. The formation of the hexameric cluster was quite sensitive to a tiny structural difference of guests; phenylethylalcohol (3), phenol (4), benzyl amine (5) and aniline (6) did not form any hexameric cluster. The packing coefficient of 0.53 suggested that the hexameric cluster nicely filled the hydrophobic space, which most likely resulted in the effective van der Waals contacts that stabilised the supramolecular organisation composed of the hexameric cluster and the polymeric array of 1 in the solid state.
(34) Chemical Functionalization and Photoluminescence of Graphene Quantum
Dots
Ryo Sekiya, Yuichiro Uemura, Hiroyoshi Naito, Kensuke Naka, and Takeharu
Haino,* Chem. Eur. J., 2016, 22, 8198-8206.
DOI:10.1002/chem.201504963
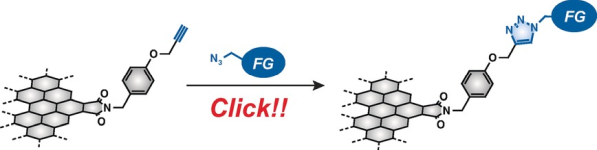
Abstract:Chemical modification of graphene quantum dots (GQDs) can influence their physical and chemical properties; hence, the investigation of the effect of organic functional groups on GQDs is of importance for developing GQD–organic hybrid materials. Three peripherally functionalised GQDs having a third-generation dendritic wedge (GQD-2), long alkyl chains (GQD-3) and a polyhedral oligomeric silsesquioxane group (GQD-4) were prepared by the CuI-catalysed Huisgen cycloaddition reaction of GQD-1 with organic azides. Cyclic voltammetry indicated that reduction occurred on the surfaces of GQD-1–4 and on the five-membered imide rings at the periphery, and this suggested that the functional groups distort the periphery by steric interactions between neighbouring functional groups. The HOMO–LUMO bandgaps of GQD-1–4 were estimated to be approximately 2 eV, and their low-lying LUMO levels (<−3.9 eV) were lower than that of phenyl-C61-butyric acid methyl ester, an n-type organic semiconductor. The solubility of GQD-1–4 in organic solvents depends on the functional groups present. The functional groups likely cover the surfaces and periphery of the GQDs, and thus increase their affinity for solvent and avoid precipitation. Similar to GQD-2, both GQD-3 and GQD-4 emitted white light upon excitation at 360 nm. Size-exclusion chromatography demonstrated that white-light emission originates from the coexistence of differently sized GQDs that have different photoluminescence emission wavelengths.
(33) Induced-fit Molecular Recognition of Alkyl Chains in p-tert-butylcalix[5]arene
in the solid state
Yasunori Kajiki, Ryo Sekiya, Yutaro Yamasaki, Yuichiro Uemura, and Takeharu
Haino,* Bull. Chem. Soc. Jpn., 2016, 89, 220-225 (Selected Paper).
DOI:10.1246/bcsj.20150344
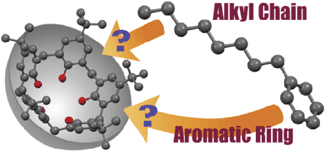
Abstract:p-tert-Butylcalix[5]arene 1 recognized conformationally flexible aliphatic hydrocarbons (hexane 2, heptane 3, octane 4, nonane 5, decane 6, undecane 7, and dodecane 8) and monoalkyl benzenes (toluene 10, ethylbenzene 11, propylbenzene 12, butylbenzene 13, hexylbenzene 14, and octylbenzne 15) to form host–guest complexes. X-ray diffraction study has revealed that the alkyl chains of 11–15 were selectively recognized by 1, whereas host 1 recognized the aromatic ring of 10. The alkyl chain termini of 3, 4, 6–8, 11–13, and 15 adopted unusual folded conformations in the cavity. M06-2X/6-31G(d,p) level of calculations revealed that the mutual induced-fit shape adjustments between the calix[5]arene cavity and the flexible guests play a key role in maximizing the host–guest interactions directed by the C(sp3)–H/π interactions, leading to the unusual conformations of the alkyl chain termini of the guests and the selective binding of the alkyl chains in the solid state.
(32) UV Photodissociation Spectroscopy of Cryogenic Cooled Gas Phase Host-guest
Complex Ions of Crown Ethers
Yoshiya Inokuchi, Takeharu Haino, Ryo Sekiya, Fumiya Morishima, Claude
Dedonder-Lardeux, Géraldine Féraud, Christophe Jouvet and Takayuki Ebata,*
Phys. Chem. Chem. Phys., 2015, 39, 25925-25934.
DOI:10.1039/C5NJ01787D
Abstract: The geometric and electronic structures of cold host–guest complex ions of crown ethers (CEs) in the gas phase have been investigated by ultraviolet (UV) fragmentation spectroscopy. As host CEs, we chose 15-crown-5 (15C5), 18-crown-6 (18C6), 24-crown-8 (24C8), and dibenzo-24-crown-8 (DB24C8), and as guests protonated-aniline (aniline·H+) and protonated-dibenzylamine (dBAM·H+) were chosen. The ions generated by an electrospray ionization (ESI) source were cooled in a quadrupole ion-trap (QIT) using a cryogenic cooler, and UV spectra were obtained by UV photodissociation (UVPD) spectroscopy. UV spectroscopy was complemented by quantum chemical calculations of the most probable complex structures. The UV spectrum of aniline·H+·CEs is very sensitive to the symmetry of CEs; aniline·H+·18C6 shows a sharp electronic spectrum similar to aniline·H+, while aniline·H+·15C5 shows a very broad structure with poor Franck–Condon factors. In addition, a remarkable cage effect in the fragmentation process after UV excitation was observed in both complex ions. In aniline·H+·CE complexes, the cage effect completely removed the dissociation channels of the aniline·H+ moiety. A large difference in the fragmentation yield between dBAM·H+·18C6 and dBAM·H+·24C8 was observed due to a large barrier for releasing dBAM·H+ from the axis of rotaxane in the latter complex.
(31) Molecular Recognition of Upper Rim Functionalized Cavitand and Its
Unique Dimeric Capsule in the Solid State
Mutsumi Kobayashi, Mei Takatsuka, Ryo Sekiya, and Takeharu Haino,* Org.
Biomol. Chem., 2015, 13, 1647-1653.
DOI:10.1039/C4OB02251C
Abstract: Cavitand 1 possesses four 2,2′-bipyridyl pillars on its upper rim that encapsulates small guests, such as nitromethane, acetonitrile, methyl acetate, ethyl acetate, and N-methylacetamide, into a deep cavity to form host–guest complexes in a 1 : 1 ratio. Nitroethane, N,N-dimethylformamide, and N,N-dimethylacetamide were not bound in this manner. A guest-binding study and molecular mechanics calculations revealed that the four 2,2′-bipyridyl pillars of cavitand 1 created a steric boundary that is responsible for selective guest recognition. In the solid state, cavitand 2 formed a unique chiral capsule 22 by π–π stacking interactions between the 2,2′-bipyridyl pillars. A nitromethane molecule was unusually placed deep inside the cavity, as directed by the multiple hydrogen bonding interactions between the nitromethane oxygen atoms, the C–H bonds of the bridge methylenes and the pillar phenyl groups.
(30) Ion-Based Assemblies of Planar Anion Complexes and Cationic PtII Complexes
Ryo Sekiya, Yusuke Tsutsui, Wookjin Choi, Tsuneaki Sakurai, Shu Seki, Yuya
Bandoa and Hiromitsu Maeda,* Chem. Commun., 2014, 50, 10615-10618.
DOI:10.1039/C4CC04565C
Abstract:Because the metallophilicity of attractive PtII⋯PtII interactions helps in the fabrication of columnar structures, terpyridine–PtII complexes were used as planar counter cationic species for formation of charge-segregated assemblies using π-conjugated receptor–Cl− complexes as planar anions.
(29) Synthesis, Characterization, X-ray Crystal Structure, DFT Calculations
and Catalytic Properties of a Dioxovanadium(V) Complex Derived from Oxamohydrazide
and Pyridoxal - A Model Complex of Vanadate Dependent Bromoperoxidase
Chandrima Das, Piyali Adak, Satyajit Mondal, Ryo Sekiya, Reiko Kuroda,
Serge Gorelsky, Shyamal Chattopadhyay, Inorg. Chem., 2014, 53, 11426-11437.
DOI:10.1021/ic501216d
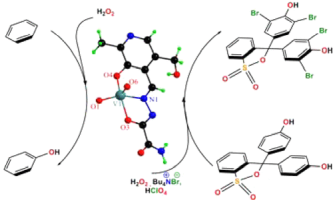
Abstract:A vanadium(V) complex with the formula [Et3NH][VVO2(sox-pydx)] with a new tridentate ligand 2-[2-[[3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl]methylene]hydrazinyl]-2-oxoacetamide (soxH-pydxH), obtained by condensation of oxamohydrazide and pyridoxal (one of the forms of vitamin B6), has been synthesized. The compound was characterized by various analytical and spectroscopic methods, and its structure was determined by single-crystal X-ray diffraction technique. Density functional theory (DFT) and time-dependent DFT calculations were used to understand the electronic structure of the complex and nature of the electronic transitions observed in UV–vis spectra. In the complex, vanadium(V) is found to be pentacoordinated with two oxido ligands and a bianionic tridentate ONO-donor ligand. The vanadium center has square-pyramidal geometry with an axial oxido ligand, and the equatorial positions are occupied by another oxido ligand and a phenolato oxygen, an imine nitrogen, and a deprotonated amide oxygen of the hydrazone ligand. A DFT-optimized structure of the complex shows very similar metrical parameters as determined by X-ray crystallography. The O4N coordination environment of vanadium and the hydrogen-bonding abilities of the pendant amide moiety have a strong resemblance with the vanadium center in bromoperoxidase enzyme. Bromination experiments using H2O2 as the oxidizing agent, with model substrate phenol red, and the vanadium complex as a catalyst show a remarkably high value of kcat equal to 26340 h–1. The vanadium compound also efficiently catalyzes bromination of phenol and salicylaldehyde as well as oxidation of benzene to phenol by H2O2.
(28) High Diastereoselection of Dissymmetric Capsule by Chiral Guest Encapsulation
Yuta Tsunoda, Katsunori Fukuta, Taisuke Imamura, Ryo Sekiya, Taniyuki Furuyama,
Nagao Kobayashi, and Takeharu Haino,* Angew. Chem. Int. Ed., 2014, 53,
7243-7247.
DOI:10.1002/anie.201403721
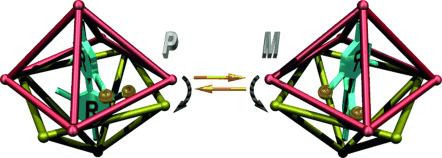
Abstract:Encapsulation of chiral guests in the dissymmetric capsule 1⋅4 BF4 formed diastereomeric supramolecular complexes G⊂1⋅4 BF4 (G: guest). When chiral guests 2 a–q were encapsulated within the dissymmetric space of the self-assembled capsule 1⋅4 BF4, circular dichroism (CD) was observed at the absorption bands that are characteristic of the π–π* transition of the bipyridine moiety of the capsule, which suggests that the P and M helicities of the capsule are biased by the chiral guest complexation. The P helicity of diastereomeric complex (S)-2 l⊂1⋅4 BF4 was determined to be predominant, based on CD exciton coupling theory and DFT calculations. The diastereoselectivity was highly influenced by the ester substituents, such that benzyl ester moieties were good for improving the diastereoselectivity. A diastereomeric excess of 98 % was achieved upon the complexation of 2 j. The relative enthalpic and entropic components for the distereoselectivity were obtained from a van’t Hoff plot. The enthalpic components were linearly correlated with the substituent Hammett parameters (σp+). The electron-rich benzyl ester moieties generated donor–acceptor π–π stacking interactions with the bipyridine moiety, which resulted in a significant difference in energy between the predominant and subordinate diastereomeric complexes.
(27) White-Light-Emitting Edge-Functionalized Graphene Quantum Dots
Ryo Sekiya, Yuichiro Uemura, Hideki Murakami, and Takeharu Haino,* Angew.
Chem. Int. Ed., 2014, 53, 5619-5623.
DOI:10.1002/anie.201311248
Abstract:Graphene quantum dots (GQDs) have received considerable attention for their potential applications in the development of novel optoelectronic materials. In the generation of optoelectronic devices, the development of GQDs that are regulated in terms of their size and dimensions and are unoxidized at the sp2 surfaces is desired. GQDs functionalized with bulky Fréchet’s dendritic wedges at the GQD periphery were synthesized. The single-layered, size-regulated structures of the dendronized GQDs were revealed by atomic force microscopy. The edge-functionalization of the GQDs led to white-light emission, which is an uncommon feature.
(26) Development of Ultraviolet-Ultraviolet Hole-burning Spectroscopy for
Cold Gas Phase Ions
Géraldine Féraud, Claude Dedonder-Lardeux, Christophe Jouvet, Yoshiya Inokuchi,
Takeharu Haino, Ryo Sekiya, Takayuki Ebata,* J. Phys. Chem. Lett., 2014,
5, 1236-1240.
DOI:10.1021/jz500478w
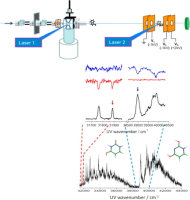
Abstract:A new ultraviolet–ultraviolet hole-burning (UV–UV HB) spectroscopic scheme has been developed for cold gas-phase ions in a quadrupole ion trap (QIT) connected with a time-of-flight (TOF) mass spectrometer. In this method, a pump UV laser generates a population hole for the ions trapped in the cold QIT, and a second UV laser (probe) monitors the population hole for the ions extracted to the field-free region of the TOF mass spectrometer. Here, the neutral fragments generated by the UV dissociation of the ions with the second laser are detected. This UV–UV HB spectroscopy was applied to protonated dibenzylamine and to protonated uracil. Protonated uracil exhibits two strong electronic transitions; one has a band origin at 31760 cm–1 and the other at 39000 cm–1. From the UV–UV HB measurement and quantum chemical calculations, the lower-energy transition is assigned to the enol–keto tautomer and the higher-energy one to the enol–enol tautomer.
(25) Guest-induced Head-to-tail Columnar Assembly of Calix[4]arene Possessing
Catechol Side Arms
Ryo Sekiya, Yutaro Yamasaki, Wataru Tada, Hidemi Shio, and Takeharu Haino,*
CrystEngComm, 2014, 16, 6023-6032.
DOI:10.1039/C4CE00667D
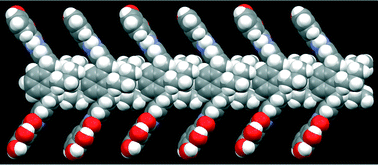
Abstract:Calix[4]arene possessing two catechol side arms 1 and organic molecules (nPrOH, AcOH, AcOEt, and CH3CN) crystallized to afford cocrystals: 1·(nPrOH)4, 1·(AcOH)4, 1·(AcOEt), and 1·(CH3CN)2. In these cocrystals, calix[4]arene 1 is arranged one-dimensionally, forming head-to-tail columnar structures. The organic guests settle in the residual space between the columnar structures; they are captured by the catechol side arms through hydrogen bonding. In the cocrystal 1·(H2O)2, a continuous zigzag array of 1 is formed instead of the columnar assembly, demonstrating that the organic guests induced the formation of the head-to-tail columnar structures in the crystal packing. The crystalline apohost 1apo was prepared by the desorption of the MeOH guests from the cocrystal 1·(MeOH)4; this compound adsorbed the MeOH vapour, reconstructing the original crystal packing. When 1apo adsorbed the iPrOH vapour, a cocrystal with a crystal structure similar to that of 1·(MeOH)4 was formed, suggesting that 1apo has a crystal structure similar to that of 1·(MeOH)4.
(24) Heteroleptic Ru(II) complexes containing aroyl hydrazone and 2,2´-bipyridyl:
Synthesis. X-ray crystal structures, electrochemical and DFT studies
Bipinbihari Ghosh, Sumita Naskar, Subhendu Naskar, Arturo Espinosa, Jacky
Yau, Thomas C. W. Mak, Ryo Sekiya, Reiko Kuroda, Shyamal Kumar Chattopadhyay,
Polyhedron, 2014, 72, 115–121.
DOI: 10.1016/j.poly.2014.01.031

Abstract:Four heteroleptic Ru(II) complexes [Ru(bpy)2(L)]X (L = bidentate N, O− donor hydrazone ligands, X = ClO4 or PF6) have been synthesized and characterized. Single crystal X-ray structures of two of the complexes are also reported. The complexes show strong absorptions in the 400–600 nm region of the visible radiation, with very similar room temperature emission spectra to that of reference [Ru(bpy)3]X2 but with the lowest energy MLCT (metal to ligand charge transfer) band bathochromically shifted by about 50 nm. Results obtained from both electrochemical experiments and DFT calculations show remarkable differences in the HOMO–LUMO properties of the hydrazone complexes compared to [Ru(bpy)3]X2. TDDFT calculations have been used to understand the nature of the electronic transitions.
(23) Head-to-tail polymeric columnar structure of calix[4]arene possessing
catechol arms in the solid state
Ryo Sekiya, Yutaro Yamasaki, Susumu Katayama, Hidemi Shio and Takeharu
Haino,* CrystEngComm, 2013, 15, 8404–8407.
DOI:10.1039/C3CE41660G
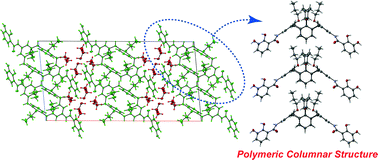
Abstract:Calix[4]arene 1 cocrystallized with methanol (MeOH), ethanol (EtOH), isopropanol (iPrOH) and 1,4-butanediol (BDO) to afford clathrate compounds 1·(MeOH)4, 1·(EtOH)4, 1·(iPrOH)4, and 1·(BDO)2, respectively. The unique head-to-tail polymeric columnar structures of 1 were found in the solid state.
(22) Anion-Directed Formation and Degradation of an Interlocked Metallohelicate
Ryo Sekiya,* Morihiko Fukuda and Reiko Kuroda,* J. Am. Chem. Soc., 2012,
134, 10987–10997.
DOI:10.1021/ja303634u
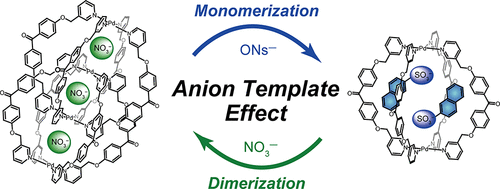
Abstract:Although there are many examples of catenanes, those of more complex mechanically interlocked molecular architectures are rare. Additionally, little attention has been paid to the degradation of such interlocked systems into their starting complexes, although formation and degradation are complementary phenomena and are equally important. Interlocked metallohelicate, [(Pd2L4)2]8+ (28+), is a quadruply interlocked molecular architecture consisting of two mechanically interlocked monomers, [Pd2L4]4+ (14+). 28+ has three internal cavities, each of which encapsulates one NO3– ion (1:3 host–guest complex, 2⊃(NO3|NO3|NO3)5+) and is characterized by unusual thermodynamic stability. However, both the driving force for the dimerization and the origin of the thermodynamic stability remain unclear. To clarify these issues, BF4–, PF6–, and OTf– have been used to demonstrate that the dimerization is driven by the anion template effect. Interestingly, the stability of 28+ strongly depends on the encapsulated anions (2⊃(NO3|NO3|NO3)5+ ≫ 2⊃(BF4|BF4|BF4)5+). The origins of this differing thermodynamic stability have been shown through detailed investigations to be due to the differences in the stabilization of the interlocked structure by the host–guest interaction and the size of the anion. We have found that 2-naphthalenesulfonate (ONs–) induces the monomerization of 2⊃(NO3|NO3|NO3)5+ via intermediate 2⊃(ONs|NO3|ONs)5+, which is formed by anion exchange. On the basis of this finding, and using p-toluenesulfonate (OTs–), the physical separation of 2⊃(NO3|NO3|NO3)5+ and 14+ as OTs– salt was accomplished.
(21) Adsorption and Separation of Poly-Aromatic Hydrocarbons by a Hydrogen-Bonded
Coordination Polymer
Ryo Sekiya*and Shin-ichi Nishikiori,* Chem. Commun., 2012, 48, 5022–5024.
DOI:10.1039/C2CC31586F
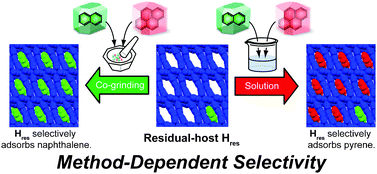
Abstract:A residual-host prepared by thermal removal of naphthalene (NA) from the inclusion compound [Ni(SCN)2(isonicotinic acid)2]·(NA)0.5 was found to function as an adsorbent for aromatic molecules and exhibit method-dependent selectivity.
(20) Pd2+···O3RS− Interaction Encourages Anion Encapsulation of a Quadruply-Stranded
Pd Complex to Achieve Chirality and High Solubility
Ryo Sekiya and Reiko Kuroda,* Chem. Commun., 2011, 47, 12346-12348.
DOI:10.1039/C1CC14982B
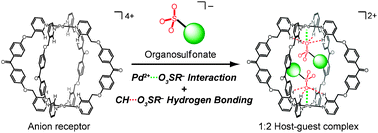
Abstract:Pd2+⋯O3SR− and CH⋯O3SR− interactions were found to play a crucial role in the encapsulation of organic sulfonates in a quadruply-stranded complex of Pd2L4-type. We have successfully employed this feature to achieve chirality induction by complexation with chiral guests as well as solubility increase by dendronization.
(19) Controlling Stereoselectivity of Solid-state Photoreactions by Co-crystal
Formation
Ryo Sekiya and Reiko Kuroda,* Chem. Commun., 2011, 47, 10097-10099.
DOI:10.1039/C1CC13484A
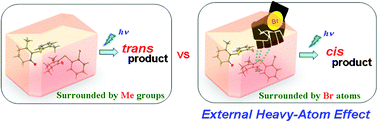
Abstract:An external heavy-atom effect in crystals was found to influence the reaction path of the photocyclization of cyclohexenones drastically, and that was successfully applied to control the cis–trans stereochemistry of the reaction by making co-crystals of heavy-atom containing and non-containing analogues in various ratios.
(18) Structural Extension from an Isonicotinic Acid Dimer to 4-(4-pyridyl)benzoic
acid (pybenH) Dimer: X-ray Crystal Structure Analysis and Inclusion Properties
of a Hydrogen-Bonded Coordination Polymer [Ni(SCN)2(pybenH)2]∞
Ryo Sekiya* and Shin-ichi Nishikiori,* Cryst. Growth Des., 2011, 11, 5574-5591.
DOI:10.1021/cg201141k
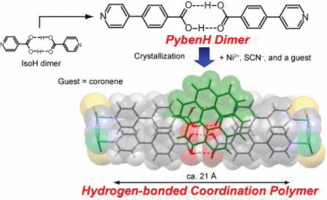
Abstract:This study reports the X-ray crystal structure analysis and inclusion properties of a hydrogen-bonded coordination polymer [Ni(SCN)2(pybenH)2]∞. The crystal structures of inclusion compounds (1–18) are best described as compounds consisting of layers. The inclusion compounds are classified into two categories: compounds with channels (channel types A (1–15) and B (16 and 17)) and compounds with isolated cavities (type I (18)). Except 18, the crystal structures are similar to each other despite different molecular structures of the guests. X-ray diffraction analysis has elucidated that the interlayer separation, the position of the cavity, and the cavity volume are adjusted to the guests. In addition, the large cavity enables the guests to adjust their positions and orientations in the channels. These backgrounds cancel out the different steric requirements of the guests to a certain extent, and, as a result, the crystal structures are similar to each other. The exceptional case of 18 is due to the molecular structure and volume of pyrene being suitable to occupy the isolated cavities formed in type I.
(17) Changing Solid-State Reaction Stereochemistry Heavy-Atom – Cocrystal
Method
Reiko Kuroda* and Ryo Sekiya, Acta Cryst. Sec. A, 2011, 67, 362.
DOI:10.1107/S010876731109088X
(16) Synthesis, X-ray Crystal Structures and Inclusion Properties of a
Hydrogen-bonded Coordination Polymer [Ni(SCN)2(pppeH)2]·(guest)x
Ryo Sekiya* and Shin-ichi Nishikiori,* CrystEngComm, 2011, 13, 6405-6414.
DOI:10.1039/C1CE05566F
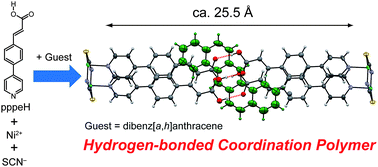
Abstract:This study reports three inclusion compounds [Ni(SCN)2(pppeH)2]·(guest)x (1–3) and a guest-free crystal [Ni(SCN)2(pppeH)2] (4), where pppeH = trans-3-[4-(4-pyridyl)phenyl]propenoic acid and guest = dibenz[a,h]anthracene (x = 1/2) (1), p-bromobenzoic acid (x = 1) (2) and 4,4′-dibromobiphenyl (x = 1) (3). The crystal structures of 1–4 are best described as consisting of layers. The layer, [Ni(SCN)2(pppeH)2]∞, is formed by R22(8) O–H⋯O hydrogen bonding between the pppeH ligands coordinating to Ni2+ ions of adjacent 1D coordination polymers. The layers of 1–3 have cavities surrounded by two pppeH dimers and four μ-1,3-SCN ligands with the dimensions of ca. 25.5 × 6.2–7.6 Å2. On the other hand, the layer of 4 does not. This indicates that the guests act as templates for the formation of the cavity. Interlayer connection of the cavities gives channels penetrating through the crystals where the guests are arranged one-dimensionally. Detailed investigation on the crystal structures of 1–3 and [Ni(SCN)2(pppeH)2]·(benz[a]anthracene)2/3 (5) reported previously has revealed that the stacking manner of the layers, the interlayer separation and the structure of the layer are adjusted in response to the guests, and simultaneously the guests adjust their positions and orientations to form a close-packed structure.
(15) Combination between Metal-Ligand Coordination and Hydrogen Bond Interaction:
A Facile Route for the Construction of 3D Coordination Networks with the
Ability to Include Relatively Large Aromatic Molecules
Ryo Sekiya,* Shin-ichi Nishikiori, and Reiko Kuroda, CrystEngComm, 2009,
11, 2251-2253.
DOI:10.1039/B911849G
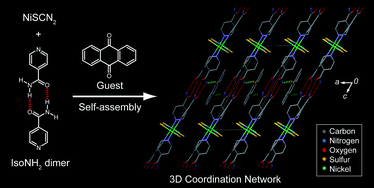
Abstract:A combination between metal–ligand coordination and hydrogen bond interaction allows the construction of new 3D coordination networks which exhibit the ability to include relatively large organic molecules.
(14) A Quadruply-Stranded Metallohelicate and Its Spontaneous Dimerization
to an Interlocked Metallohelicate
Morihiko Fukuda, Ryo Sekiya,* and Reiko Kuroda,* Angew. Chem. Int. Ed.,
2008, 47, 706-710.
DOI:10.1002/anie.200703162
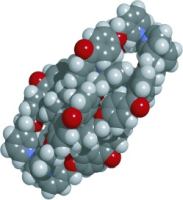
Abstract:Self-assembly of 4,4′-(3-pyridinemethoxy)benzophenone (L) and Pd(NO3)2 resulted in the quantitative formation of a quadruply stranded metallohelicate [Pd2(L)4], which undergoes spontaneous dimerization to an unprecedented chiral interlocked metallohelicate [Pd2(L)4]2 (see X-ray structure; C black, H white, N blue, O red).
(13) Decelerated Chirality Interconversion of an Optically Inactive 310-Helical
Peptide by Metal Chelation
Naoki Ousaka, Norihiko Tani, Ryo Sekiya, and Reiko Kuroda,* Chem. Commun.,
2008, 44, 2894-2896.
DOI:10.1039/B803080D
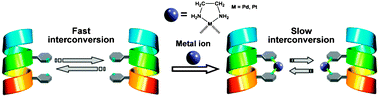
Abstract:A dynamically optically inactive 310-helical peptide possessing metal chelating ability between the side-chains at the i and i + 3th residues was synthesized, and its metal complexes were shown to stabilize the peptide structure and the helical sense.
(12) Chirality Realized Only in the Crystalline State: Inorganic and Organic
Compounds
Reiko Kuroda,* Takunori Harada, and Ryo Sekiya, Acta Cryst. Sec. A, 2008,
64, 34.
DOI:10.1107/S0108767308098930
(11) Coordination Framework Hosts Consisting of 4-Pyridyl-Substituted Carboxylic
Acid (PCA) Dimers and 1D Chains of Ni2+ and SCN-: A Rational Structural
Extension toward Coordination Framework Hosts with Large Rectangular Cavities
Ryo Sekiya* and Shin-ichi Nishikiori,* Inorg. Chem., 2006, 35, 9233-9244.
DOI:10.1021/ic0605367
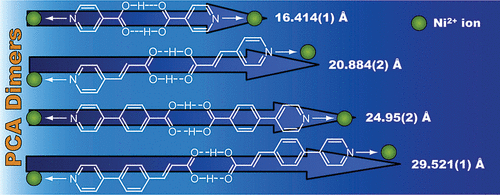
Abstract:For the expansion of a rectangular cavity (RC) defined by two isonicotinic acid (isoH) dimers as bridging ligands and two SCN bridges, we conducted a structural extension based on the elongation of the bridging ligands by the replacement of isoH with longer 4-pyridyl-substituted carboxylic acid (PCA). For this purpose, the following three PCAs have been employed: trans-3-(4-pyridyl)propenoic acid (acrylH), 4-(4-pyridyl)benzoic acid (pybenH), and trans-3-(4-(4-pyridyl)phenyl)propenoic acid (pppeH). Self-assembly of Ni2+, SCN-, and each of four PCAs involving isoH, acrylH, pybenH, and pppeH in the presence of an aromatic guest gave four inclusion compounds formulated as [Ni(SCN)2(isoH)2]·1/2(benz[a]anthracene) (1), [Ni(SCN)2(acrylH)2]·1/2(benz[a]anthracene) (2), [Ni(SCN)2(pybenH)2]·(pyrene) (3), and [Ni(SCN)2(pppeH)2]3/2·(benz[a]anthracene) (4). X-ray crystal structural determination of 1−4 revealed that the proposed structural extension was successful. Their crystal structures are layered structures of two-dimensional (2D) grid-type coordination frameworks (2D host layers) framed with bridging ligands of the corresponding PCA dimers and 1D chains consisting of Ni2+ ions and μ1,3-SCN- ions. The lengths of the PCA dimers are 12.269(5) Å (isoH dimer), 16.890(4) Å (acrylH dimer), 20.89(2) Å (pybenH dimer), 25.387(3) Å (pppeH dimer A), and 25.527(4) Å (pppeH dimer B). Each 2D host layer has RCs defined by the two corresponding PCA dimers and the two SCN bridges. The dimensions of RCs are expanded in proportion to the increase in the lengths of the PCA dimers: 29.52 × 5.60−7.20 Å2 (4) > 24.95 × 5.46−7.38 Å2 (3) > 20.88 × 5.49−7.25 Å2 (2) > 16.41 × 5.53−7.43 Å2 (1). These expansions reflect the number of aromatic guests that can be included in RCs. RC of 1 include only one molecule of benz[a]anthracene, whereas RCs of 3 or 4 includes two molecules of pyrene or benz[a]anthracene, respectively. Comparison of the lengths between the PCA dimers and 4,4‘-bipyridine-type ligands demonstrated that a design strategy−the preparation of a bridging ligand through self-assembly of two PCAs−is both efficient and particularly suitable for the preparation of very long bridging ligands.
(10) 4-(4-pyridyl)benzoic Acid (PybenH) Dimer: An Efficient and Reasonable
Design for a Long Linear Bidentate Building Block Employed in Metal-Organic
Coordination Framework
Ryo Sekiya* and Shin-ichi Nishikiori,* Chem. Lett., 2006, 35, 614-615.
DOI:10.1246/cl.2006.614
Abstract:Self-assembly of 4-(4-pyridyl)benzoic acid (pybenH), Ni2+, SCN− and an aromatic guest (naphthalene or dibenz[a,h]anthracene) gave two isostructural inclusion compounds, in which pybenH ligands form the corresponding dimers acting as bidentate building blocks with a length of ca. 20.86 Å.
(9) Cu3(CN)4(NH3)2Hg(CN)2: a Novel Interpenetrating Framework Formed From
CuI, CuII, HgII and Cyanide Bridges
Shin-ichi Nishikiori,* Ryo Sekiya, and Kazumasa Hosoya, Acta Cryst., 2006,
C62, 32-34.
DOI:10.1107/S0108270106011711
Abstract:The title compound, poly[diamminehexa-mu-cyano-dicopper(I)copper(II)mercury(II)], [Cu3Hg(CN)6(NH3)2]n, has a novel threefold-interpenetrating structure of three-dimensional frameworks. This three-dimensional framework consists of two-dimensional network Cu3(CN)4(NH3)2 complexes and rod-like Hg(CN)2 complexes. The two-dimensional network complex contains trigonal-planar Cu(I) (site symmetry m) and octahedral Cu(II) (site symmetry 2/m) in a 2:1 ratio. Two types of cyanide group form bridges between three coordination sites of Cu(I) and two equatorial sites of Cu(II) to form a two-dimensional structure with large hexagonal windows. One type of CN- group is disordered across a center of inversion, while the other resides on the mirror plane. Two NH3 molecules (site symmetry 2) are located in the hexagonal windows and coordinate to the remaining equatorial sites of Cu(II). Both N atoms of the rod-like Hg(CN)2 group (Hg site symmetry 2/m and CN- site symmetry m) coordinate to the axial sites of Cu(II). This linkage completes the three-dimensional framework and penetrates two hexagonal windows of two two-dimensional network complexes to form the threefold-interpenetrating structure.
(8) A Hofmann Pyridine Complex: poly[tetra-μ-cyano-dipyridinemanganese(II)nickel(II)]
Kazumasa Hosoya, Ryo Sekiya, and Shin-ichi Nishikiori,* Acta Cryst., 2006,
E26, 1627-1629.
DOI:10.1107/S1600536806022057
Abstract:The title compound, [MnNi(CN)4(C5H5N)2], has a layered structure of a two-dimensional network complex Mn(py)2Ni(CN)4, in which linkages of cyanide between the equatorial coordination sites of octahedral MnII ions (site symmetry 2/m) and those of square planar NiII ions (site symmetry 2/m) form a square mesh network. Pyridine ligands (located on mirror planes) coordinate to the axial sites of the MnII ions from both sides of the network.
(7) A New Inclusion Compound Consisting of 2D Coordination Layers of [Cd(SCN)2]∞
and Pillars of Isonicotinamide Dimers
Ryo Sekiya* and Shin-ichi Nishikiori,* Chem. Lett., 2005, 34, 1076-1077.
DOI:10.1246/cl.2005.1076
Abstract:Self-assembly of Cd2+, SCN−, isonicotinamide (isoNH2), and 9,10-dichloroanthracene gave a new inclusion compound formulated as [Cd(SCN)2(isoNH2)2]·1/2(9,10-dichloroanthracene) (1). This inclusion compound consists of 2D coordination layers of [Cd(SCN)2]∞ and pillars of isoNH2 dimers. 9,10-dichloroanthracene molecules are included in zigzag channels.
(6) Crystalline Inclusion Compounds Constructed through Self-Assembly of
Isonicotinic Acid and Thiocyanato Coordination Bridges
Ryo Sekiya,* Shin-ichi Nishikiori, Katuyuki Ogura, J. Am. Chem. Soc., 2004,
127, 16587-16600.
DOI:10.1021/ja0463280
Abstract:The synthesis, crystal structures, inclusion ability, and structural robustness of novel crystalline inclusion compounds of [Ni(SCN)2(isoH)2]·xG (isoH = isonicotinic acid; G = aromatic guest) are described. The inclusion compounds are constructed by stacking identical 2D host layers that consist of SCN-, isoH, and Ni2+ with van der Waals contact separation. In the layer, two types of rectangular cavities (A-type and B-type) are formed, and the guests are included in the former cavity. The inclusion compounds were categorized into four stacking modes according to the difference in the stacking mode of the layers. A systematic investigation of the crystal structures of the 21 inclusion compounds clarified the close relationship between the molecular structure of the guest and the resultant stacking mode of the layers.
(5) Design and Structural Extension of a Supramolecular Inclusion Compound
Host Built with Combination of Dimer Formation of Isonicotinic Acid and
Thiocyanato Coordinating Bridges
Ryo Sekiya and Shin-ichi Nishikiori,* Chem. Eur. J., 2002, 8, 4803-4810.
DOI:10.1002/1521-3765(20021018)8:20<4803::AID-CHEM4803>3.0.CO;2-H
Abstract:A new host design for an inclusion compound with a preference for large planar aromatic guest molecules has been proposed. Our host design includes a rectangular cavity made using a long and a short building block based on the concept of supramolecular chemistry. The long building block facilitates the inclusion of large guests, and the short building block prevents the formation of an interpenetrated structure, which is often observed in frameworks with large void spaces. The long building block is made when dimers of 4-pyridinecarboxylic acid (isoH) form through hydrogen bonding between the two carboxylic acid moieties. This isoH dimer can link two transition metal centers using the N atoms at both ends to act as a long building block. For the short building block, the thiocyanato ion was used. This makes a bent bridge between two metal centers to form a 1D double-chain [M(SCN)2]∞ complex. From the self-assembly of isoH, SCN− and Ni2+, a 2D network of [Ni(SCN)2(isoH)2]∞, in which the 1D [Ni(SCN)2]∞ complexes are linked by the isoH dimers, is built up. The rectangular cavity is formed as a mesh within the 2D network. The crystal of our inclusion compound has a layered structure of 2D networks, and a 1D channel-like cavity penetrating the layered 2D networks is formed where guests may be included. Moreover, our host design has the advantage of easy extension of the host structure. Replacement of isoH with another component and use of three components is possible for making the long building block. In the latter case, a linear spacer having two carboxy groups is inserted into the isoH dimer to form a long building block with a trimer structure. Based on our host design, a series of new inclusion compounds were synthesized. The crystal structures of three compounds were determined by single crystal X-ray diffraction. These were a biphenyl inclusion compound [Ni(SCN)2(isoH)2]⋅1/2C12H10 (the basic case), a 9,10-dichloroanthracene inclusion compound [Ni(SCN)2(acrylH)2]⋅1/2C14H8Cl2, where isoH is replaced with 3-(4-pyridinyl)-2-propenoic acid (acrylH), and a perylene inclusion compound [Ni(SCN)2(isoH)2(fumaricH2)]⋅1/2C20H12, whose long building block is a trimer inserted with fumaric acid (fumaricH2) as a linear spacer.
(4) A Preparative Strategy for Supramolecular Inclusion Compounds by Combination
of Dimer Formation of Isonicotinic Acid and Coordination Bonding
Ryo Sekiya and Shin-ichi Nishikiori,* Chem. Commun., 2001, 2612-2613.
DOI:10.1039/B108458E
Abstract:A new inclusion compound host [Ni(SCN)2(isoH)2], whose cavity is suitable to include large aromatic guests, has been synthesized by a method of combining the dimer formation of isonicotinic acid by double hydrogen bonds with a 1D Ni thiocyanato complex.
(3) Microwave Spectroscopic and ab initio studies of pyrolysis products
of 2-nitrosopropene (syn form) and its pyrolysis mechanism
Takeshi Sakaizumi,* Hirotaka Imajo, Ryo Sekiya, Nobuhiko Kuze, and Osamu
Ohashi, J. Anal. App. Pyrolysis, 2001, 60, 131-144.
DOI:10.1016/S0165-2370(00)00165-0
Abstract:The pyrolysis products of CH2C(CH3)NO (syn form) have been determined by microwave spectroscopy. The pyrolysis products of CH2C(CH3)NO (syn form) and its 15N isotopic species were found to be CH2O, CH3CN, and CH3C15N. The produce of formaldehyde and methyl cyanide suggests that the CC and NO double bonds of CH2C(CH3)NO (syn form) were broken. To explain the generation of the two molecules, a four-membered ring molecule (9) as an intermediate, is proposed. The four-membered ring molecule as an intermediate is also supported by ab initio MO calculation. Applying the pyrolysis mechanism obtained for 2-nitrosopropene (syn form) to that of 1,1,2-trichloronitrosoethane, one of its complicated pyrolysis mechanisms was explained. The rotational constants and geometrical parameters of two intermediates, 9 and CH2CClNO (13), were obtained by ab initio MO calculation (MP2/6-31G**) to predict their microwave spectra.
(2) Intramolecular Migration of Bulky Substituents in the Solid State:
Vinylogous Pinacol Rearrangements Induced Thermally and by Acid Catalysis
Ryo Sekiya, Kazuhiko Kiyo-oka, Tatsuro Imakubo, and Keiji Kobayashi,* J.
Am. Chem. Soc., 2000, 122, 10282-10288.
DOI:10.1021/ja000788l
Abstract:A vinylogous pinacol rearrangement, involving an intramolecular 1,4-migration of the bulky thienothienyl substituent and a marked change of the crystal structure, was thermally induced in the solid state for thienothienyl-substituted 9,10-dihydroxy-9,10-dihydroanthracenes, 1 and 3∼8. When heated over 180 to ∼240 °C, these crystals were transformed quantitatively, accompanying dehydration, to crystals of the anthrone derivatives without wetting or melting. Diol 8, which bears two isomeric thienothiophene rings, afforded only a single anthrone product. Compounds 1 and 8 formed a mixed crystal and its thermally induced reaction yielded two anthrone products that were attributable to intramolecular migration but afforded no anthrone derivative having the two substituents possible for the intermolecular combination. These results, along with the X-ray crystal structure of the mixed crystal, demonstrate that the rearrangement in the solid state proceeds intramolecularly. The 1,4-pinacol type rearrangements were also induced, along with the cis−trans isomerization, by cogrinding the diols with p-toluenesulfonic acid in a mortar at room temperature. The intramolecularity of the rearrangement was again proved by the use of the mixed crystal. The intervention of the carbocation in the solid-state grinding was ascertained by the UV/vis spectra. The time courses of the product distributions showed discrete profiles between the trans and cis diols, 1 and 3, respectively; for the trans diol 1, fast isomerization to the cis isomer and consecutive formation of the rearranged product via the cis isomer were observed. On the basis of these observations, the solid-state 1,4-rearrangement was deduced to occur preferentially via the cis configuration of the dihydroxy groups.
(1) Mass and Microwave Spectroscopic Studies of Pyrolysates and Pyrolysis
Mechanism of 1,1,2-trichloronitrosoethane
Takeshi Sakaizumi,* Ryo Sekiya, Nobuhito Kuze, and Osamu Ohashi, J. Anal.
App. Pyrolysis, 2000, 53, 177-184.
DOI:10.1016/S0165-2370(99)00065-0
Abstract:The pyrolysates of 1,1,2-trichloronitrosoethane (CH2ClCCl2NO) have been investigated by pyrolysis–mass spectrometry and microwave spectroscopy (Py–MS/MW). The five pyrolysates were identified to be 1,1-dichloroethene 1, nitrosyl chloride 2, chloroacetylene 3, formaldehyde 6 and cyanogen chloride 7 by microwave spectroscopy. The generation of 3 was verified by the direct reaction of 1 with NO* and Cl* radicals inside a Stark cell. Two radicals were generated by pyrolysis of 2. The observed ionic peaks m/z at 91 and 93 (C2H2ClNO) having the relative intensity of 3:1 were tentatively assigned to 1-chloronitrosoethene (4 or 4′) produced by elimination of Cl2 of the precursor. Two pyrolysis mechanisms of 1,1,2-trichloronitrosoethane were elucidated by judging from these pyrolysates identified. One is that 1 is generated by elimination of 2, and 3 is furthermore produced by reaction of 1 with NO and Cl radicals. The other is that 4 may be generated by elimination of Cl2 of the precursor and 6 and 7 may be produced by cleavage of four-membered ring molecule 5 produced by intramolecular cyclization of 4.
日本語解説
「トップダウン法により得られるナノグラフェンの有機化学」関谷亮*・灰野岳晴*
化学と工業2021年6月号p409-411,特集「ナノカーボン材料はどこまで進んだか?」(日本化学会)
「(ナノ)グラフェン」関谷亮*・灰野岳晴*有機合成化学会誌2021年8月p.792.
「トップダウン法により得られる化学修飾ナノグラフェン」関谷亮*・灰野岳晴*
ファインケミカル2020年1月号(シーエムシー出版)解説論文(依頼)