Introduction
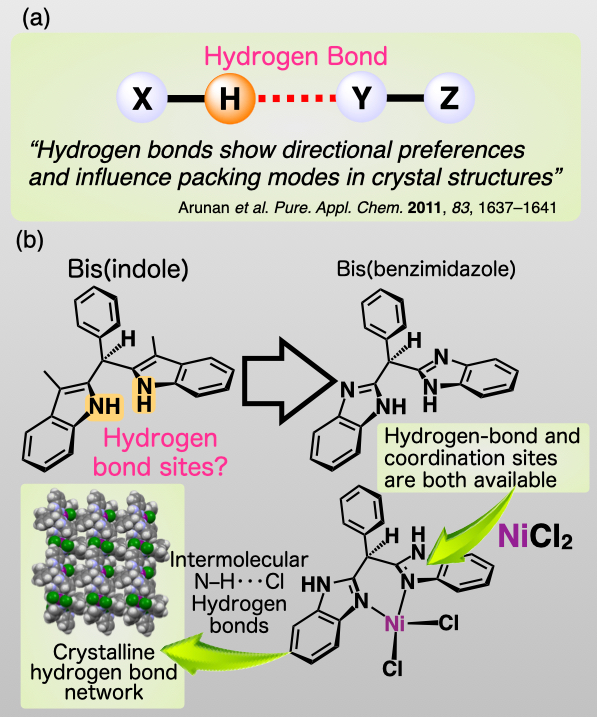
In a textbook for high-school students, "hydrogen bond" is defined to "the intermolecular force ocurring when a hydrogen atom that is bonded to a highly electronegative atom of one molecule is attracted to two unshared electrons of another molecule". Due to hydrogen bonds, the boiling points of water and ammonia are understood to be extremely higher than those expected from their molecular weights. Another feature of hydrogen bond is directionality. This feature influeces packing modes in crystal structure; thus, hydrogen bond is useful to construct remarkable crystal structures(Fig.1(a)).From this perspective, we are focusing on the construction of unique crystalline hydrogen bond networks based on coordination compounds.
[References] Sarquis J. L. et al."Modern Chemistry"(Link)
Arunan, E. et al. Pure Appl. Chem. 2011, 83, 1637–1641 (Link)
Arunan, E. et al. Pure Appl. Chem. 2011, 83, 1637–1641 (Link)
Fig.1 : (a)Directionality of hydrogen bond。(b)Concept of this study
Concept
This study was initiated by the envision that the N–H sites of bis(indole) may be useful to synthesize hydrogen-bonded networks(Fig.1(b)). However, the coordination sites of bis(indole) are lost if the N–H sites are used for hydrogen bonds, and the coordination of the ligand with the metal center is required to avoid random orientation of the two indolyl groups. Thus, we subsequently focused on bis(benzimidazole), which provides coordination sites as well as hydrogen-bond sites. Bis(benzimiazole)-coordinated metal halides should construct the crystalline hydrogen bond networks by the intermolecular N–H···Cl hydrogen bonds.Achievements
Base on the concept described above, we analyzed a crystal structure of a bis(benzimidazole)-coordinated nickel dichlorido complex, which includes diethyl ether as shown in Fig.2(a), and found that the nickel complexes form a 2D sheet structure by the intermolecular N–H···Cl hydrogen bonds and π-π interactions (Fig.2(b)). Moreove, we revealed that the phenyl groups attached to the carbon atom linking the two benzimidazoles protrude alternately from the 2D sheets to form 1D channels (Fig.2(c)). These 1D channels are occupied by the co-crystallized diethyl ether molecules.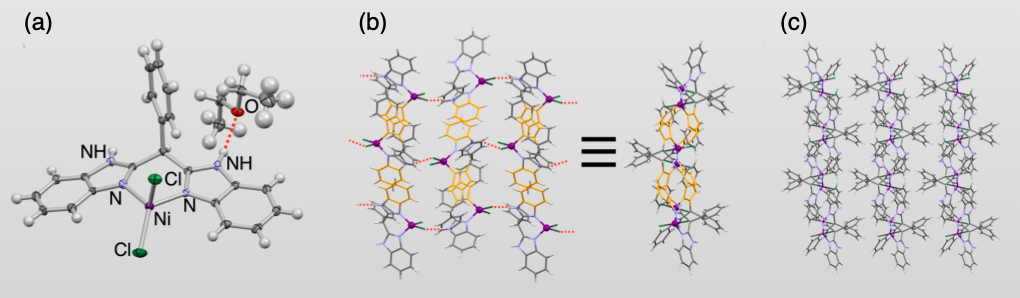
The location of the diethyl ether molecules within the 1D channels prompted us to attempt to remove diethyl ethers. For that purpose, the crystalline samples were heated under reduced pressure to afford the ether-removed crystals (Fig.3(top)). It was also found the ether-removed crystals adsorb the vapor of diethyl ether. We further revealed the mechanism of adsorption and desorption processes on the molecular levels by comparing the structures between the adsorbed/desorbed states (Fig. 3(bottom)).
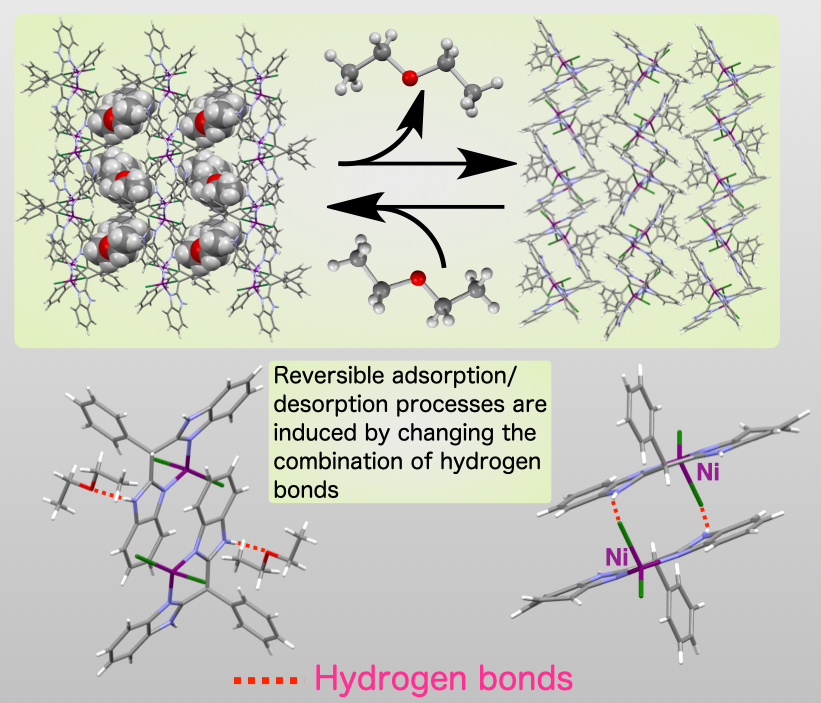
Fig.3 : Reversible adsorption and desorption of diethyl ether.

